Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems
Abstract
:1. Introduction
2. Impact of Particle Size
2.1. Impact of Particle Size on Systemic Drug Delivery
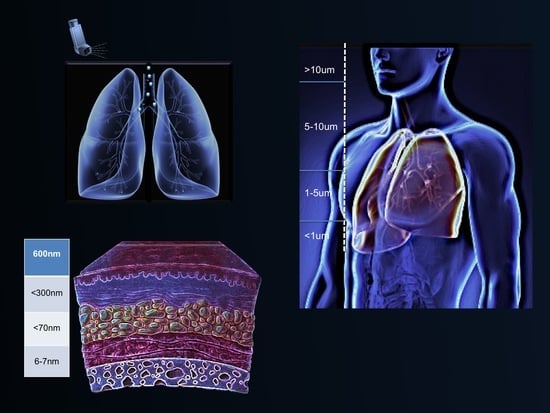
2.2. Impact of Particle Size on Pulmonary Drug Delivery
2.3. Impact of Particle Size on Drug Delivery to Tumours
2.4. Impact of Particle Size on Transdermal Drug Delivery
2.5. Impact of Particle Size on Drug Delivery to Brain
3. Polydispersity Index
4. Methods of Analysis
5. Conclusions
Funding
Conflicts of Interest
References
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Mortazavi, S.M. Nanoliposomes: From Fundamentals to Recent Developments; Trafford Pub. Ltd.: Oxford, UK, 2005. [Google Scholar]
- Sharma, D.; Ali, A.A.; Trivedi, L.R. An Updated Review on: Liposomes as drug delivery system. PharmaTutor 2018, 6, 50–62. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Poly. Mater. Poly. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Tian, W.; Schulze, S.; Brandl, M.; Winter, G. Vesicular phospholipid gel-based depot formulations for pharmaceutical proteins: Development and in vitro evaluation. J. Control. Release 2010, 142, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Breitsamer, M.; Winter, G. Needle-free injection of vesicular phospholipid gels—A novel approach to overcome an administration hurdle for semisolid depot systems. J. Pharm. Sci. 2017, 106, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Danaei, M.; Javanmard, R.; Raji, M.; Maherani, B. Nanoscale Lipidic Carrier Systems: Importance of Preparation Method and Solvents. Glob. J. Nano 2017, 2, 555593. [Google Scholar]
- Nomura, S.I.; Yoshikawa, Y.; Yoshikawa, K.; Dannenmuller, O.; Chasserot-Golaz, S.; Ourisson, G.; Nakatani, Y. Towards Proto-Cells: “Primitive” Lipid Vesicles Encapsulating Giant DNA and Its Histone Complex. ChemBioChem 2001, 2, 457–459. [Google Scholar] [CrossRef]
- Monnard, P.A.; Luptak, A.; Deamer, D.W. Models of primitive cellular life: Polymerases and templates in liposomes. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Abd-Manap, M.Y. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J. Liposome Res. 2014, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Yazid, A.M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food. Sci. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Maherani, B.; Wattraint, O. Liposomal structure: A comparative study on light scattering and chromatography techniques. J. Dispers. Sci. Technol. 2017, 38, 1633–1639. [Google Scholar] [CrossRef]
- Aveling, E.; Zhou, J.; Lim, Y.F.; Mozafari, M.R. Targeting lipidic nanocarriers: Current strategies and problems. Pharmakeftiki 2006, 19, 101–109. [Google Scholar]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Bahari, L.A.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Sadat, S.M.; Jahan, S.T.; Haddadi, A. Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J. Biomater. Nanobiotechnol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Leroux, J.C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Townsley, M.I.; Parker, J.C.; Longenecker, G.L.; Perry, M.L.; Pitt, R.M.; Taylor, A.E. Pulmonary embolism: Analysis of endothelial pore sizes in canine lung. Am. J. Physiol. Heart Circ. Physiol. 1988, 255, H1075–H1083. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ota, Z.; Shikata, K.; Hironaka, K.; Hayashi, Y.; Ota, K.; Kushiro, M.; Miyatake, N.; Kishimoto, N.; Makino, H. High-resolution ultrastructural comparison of renal glomerular and tubular basement membranes. Am. J. Nephrol. 1999, 19, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Development of non-toxic liposomal formulations for gene and drug delivery to the lung. Technol. Health Care 2002, 10, 342–344. [Google Scholar]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, T.R. Pathophysiological and disease constraints on aerosol deposition. In Respiratory Drug Delivery; Byron, P.R., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 1–38. [Google Scholar]
- Effros, R.M. Measurements of pulmonary epithelial permeability in vivo. Am. Rev. Respir. 1983, 127, S59–S65. [Google Scholar]
- Folkesson, H.G.; Westrom, B.R.; Karlsson, B.W. Permeability of the respiratory tract to different-sized macromolecules after intratracheal instillation in young and adult rats. Acta Physiol. 1990, 139, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Rytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Exp. Opin. Drug Deliv. 2008, 5, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Gaul, R.; Ramsey, J.M.; Heise, A.; Cryan, S.A.; Greene, C.M. Nanotechnology approaches to pulmonary drug delivery: Targeted delivery of small molecule and gene-based therapeutics to the lung. In Design of Nanostructures for Versatile Therapeutic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 221–253. [Google Scholar]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Pardakhty, A.; Azarmi, S.; Jazayeri, J.A.; Nokhodchi, A.; Omri, A. Role of nanocarrier systems in cancer nanotherapy. J. Liposome Res. 2009, 19, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Aw-Yong, P.Y.; Gan, P.H.; Sasmita, A.O.; Mak, S.T.; Ling, A.P. Nanoparticles as carriers of phytochemicals: Recent applications against lung cancer. Int. J. Res. Biomed. Biotechnol. 2018, 7, 1–11. [Google Scholar]
- Greish, K.; Fang, J.; Inutsuka, T.; Nagamitsu, A.; Maeda, H. Macromolecular therapeutics. Clin. Pharmacokinet. 2003, 42, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, G. Clinically approved liposomal nanomedicines: Lessons learned from the biomolecular corona. Nanoscale 2018, 10, 4167–4172. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Barenholz, Y.; Bialer, M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: Pharmacokinetic studies in rodents and dogs. Pharm. Res. 1993, 10, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, R.T.; Williams, L.E.; Presant, C.A.; Tin, G.W.; Uliana, J.A.; Gamble, R.C.; Baldeschwieler, J.D. Tumor-imaging potential of liposomes loaded with In-111-NTA: Biodistribution in mice. J. Nucl. Med. 1983, 24, 45–51. [Google Scholar] [PubMed]
- Rocha, M.; Chaves, N.; Bao, S. Nanobiotechnology for Breast Cancer Treatment. In Breast Cancer-From Biology to Medicine; InTech: Winchester, UK, 2017. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Dev. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, F.; Fei, X.; Wu, S.; Wu, D.; Pan, M.; Luo, S.; Gu, N.; Dou, J. PEGylated long-circulating liposomes deliver homoharringtonine to suppress multiple myeloma cancer stem cells. Exp. Biol. Med. 2017, 242, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Merkel, O.M.; Kissel, T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J. Control. Release 2012, 161, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Gagnadoux, F.; Hureaux, J.; Vecellio, L.; Urban, T.; Le Pape, A.; Valo, I.; Montharu, J.; Leblond, V.; Boisdron-Celle, M.; Lerondel, S.; et al. Aerosolized chemotherapy. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Mezei, M.; Gulasekharam, V. Liposomes—A selective drug delivery system for the topical route of administration, I. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef]
- Mezei, M.; Gulasekharam, V. Liposomes—A selective drug delivery system for the topical route of administration: II. gel dosage form. J. Pharm. Pharmacol. 1982, 34, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.F.; Ganesan, M.G.; Weiner, N.D.; Flynn, G.L. Mechanisms of topical delivery of liposomally entrapped drugs. J. Control. Release 1985, 2, 61–65. [Google Scholar] [CrossRef]
- Vanic, Z.; Holaeter, A.M.; Skalko-Basnet, N. (Phospho) lipid-based nanosystems for skin administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.; Ramachandran, C.; Weiner, N.; Muller, D.G. The influence of particle size of liposomes on the deposition of drug into skin. Int. J. Pharm. 1994, 103, 277–282. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: A skin penetration and confocal laser scanning microscopy study. Eur. J. Pharm. Biopharm. 2003, 55, 271–277. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Geusens, B.; Strobbe, T.; Bracke, S.; Dynoodt, P.; Sanders, N.; Van Gele, M.; Lambert, J. Lipid-mediated gene delivery to the skin. Eur. J. Pharm. Sci. 2011, 43, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, O.S.; Kim, H.S.; Cha, J.H.; Kim, H.S.; Kim, J.K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813. [Google Scholar]
- Benson, H.A. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Kumar, F. Transfersomes: Ultradeformable vesicles for transdermal drug delivery. Asian J. Biomater. Res. 2017, 3, 1–3. [Google Scholar]
- Yang, L.; Wu, L.; Wu, D.; Shi, D.; Wang, T.; Zhu, X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int. J. Nanomed. 2017, 12, 3357. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Ahmed, N.; Shah, K.U.; Khan, G.M.; Rehman, A.U. Functionalised nanostructures for transdermal delivery of drug cargos. J. Drug Target. 2018, 26, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Javanmard, R.; Raji, M. Tocosome: Novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.D.; Sravani, A.B.; Ravisankar, V.; Prakash, P.R.; Reddy, Y.S.; Bhaskar, N.V. Transferosomes a novel vesicular carrier for transdermal drug delivery system. J. Innov. Pharm. Biol. Sci. 2015, 2, 193–208. [Google Scholar]
- Lesniak, M.S.; Brem, H. Targeted therapy for brain tumours. Nat. Rev. Drug Discov. 2004, 3, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Trompetero, A.; Gordillo, A.; del Pilar, M.C.; Cristina, V.; Bustos Cruz, R.H. Alzheimer’s Disease and Parkinson’s Disease: A Review of Current Treatment Adopting a Nanotechnology Approach. Curr. Pharm. Des. 2018, 24, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Drug targeting. Breaking down barriers. Science 2002, 297, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.N.; Wang, G.L.; Hei, Y.; Meng, S.; Yang, L.F.; Yuan, L.; Xie, Y. A dual-mediated liposomal drug delivery system targeting the brain: Rational construction, integrity evaluation across the blood–brain barrier, and the transporting mechanism to glioma cells. Int. J. Nanomed. 2017, 12, 2407–2425. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.D.; Tchung, E.; Nowell, C.; Kaga, S.; Leong, N.; Mehta, D.; Kaminskas, L.M.; Boyd, B.J. Microfluidic preparation of drug-loaded PEGylated liposomes, and the impact of liposome size on tumour retention and penetration. J. Liposome Res. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nobbmann, U.L. Polydispersity–What Does It Mean for DLS and Chromatography. 2014. Available online: http://www.materials-talks.com/blog/2014/10/23/polydispersity-what-does-it-mean-for-dls-and-chromatography/ (accessed on 14 March 2018).
- Bera, B. Nanoporous silicon prepared by vapour phase strain etch and sacrificial technique. In Proceedings of the International Conference on Microelectronic Circuit and System (Micro), Kolkata, India, 11–12 July 2015; pp. 42–45. [Google Scholar]
- Worldwide, M.I. Dynamic Light Scattering, Common Terms Defined; Inform White Paper; Malvern Instruments Limited: Malvern, UK, 2011; pp. 1–6. [Google Scholar]
- Rane, S.S.; Choi, P. Polydispersity index: How accurately does it measure the breadth of the molecular weight distribution? Chem. Mater. 2005, 17, 926. [Google Scholar] [CrossRef]
- Gooch, J.W. Polydispersity. In Encyclopedic Dictionary of Polymers; Springer: New York, NY, USA, 2011; p. 556. [Google Scholar]
- Clarke, S. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Ph.D. Thesis, Dublin City University, Northside, Dublin, 2013. [Google Scholar]
- Badran, M. Formulation and in vitro evaluation of flufenamic acid loaded deformable liposome for improved skin delivery. Digest J. Nanomater. Biostruct. 2014, 9, 83–91. [Google Scholar]
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Putri, D.C.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposome preparation. J. Pharm. Sci. Commun. 2017, 14, 79–85. [Google Scholar] [CrossRef]
- FDA—Liposome Drug Products; Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; Labeling Documentation. Guidance for Industry; April 2018 Pharmaceutical Quality/CMC.; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2018.
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.; Chow, A.H. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Ozer, A.Y. Applications of light and electron microscopic techniques in liposome research. In Nanomaterials and Nanosystems for Biomedical Applications; Springer: Dordrecht, The Netherlands, 2007; pp. 145–153. [Google Scholar]
- Jones, M.N. The surface properties of phospholipid liposome systems and their characterisation. Adv. Colloid Interface Sci. 1995, 54, 93–128. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Reed, C.J.; Rostron, C.; Hasirci, V. A review of scanning probe microscopy investigations of liposome-DNA complexes. J. Liposome Res. 2005, 15, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Prospects of anionic nanolipoplexes in nanotherapy: Transmission electron microscopy and light scattering studies. Micron 2007, 38, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Negussie, A.H.; Yarmolenko, P.S.; Partanen, A.; Ranjan, A.; Jacobs, G.; Woods, D.; Bryant, H.; Thomasson, D.; Dewhirst, M.W.; Wood, B.J.; et al. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int. J. Hyperth. 2011, 27, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. In Liposomes; Humana Press: New York, NY, USA, 2010; pp. 29–50. [Google Scholar]
- Jiang, Y.; Genin, G.M.; Pryse, K.M.; Elson, E.L. Atomic force microscopy of phase separation on ruptured, giant unilamellar vesicles. BioRxiv 2018, 250944. [Google Scholar] [CrossRef]
- Hasegawa, Y. Scanning Tunneling Microscopy. In Compendium of Surface and Interface Analysis; Springer: Singapore, 2018; pp. 599–604. [Google Scholar]
- Khosravi-Darani, K.; Pardakhty, A.; Honarpisheh, H.; Rao, V.M.; Mozafari, M.R. The role of high-resolution imaging in the evaluation of nanosystems for bioactive encapsulation and targeted nanotherapy. Micron 2007, 38, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Putnam, C.D.; Hammel, M.; Hura, G.L.; Tainer, J.A. X-ray solution scattering (SAXS) combined with crystallography and computation: Defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007, 40, 191–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, S.; Huang, T.; Wang, S.; Yan, X. Analytical techniques for single-liposome characterization. Anal. Methods 2013, 5, 2150–2157. [Google Scholar] [CrossRef]
- Henriquez, R.R.; Ito, T.; Sun, L.; Crooks, R.M. The resurgence of Coulter counting for analyzing nanoscale objects. Analyst 2004, 129, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Broom, M.F.; Tucker, I.G. Characterization of a nanoparticulate drug delivery system using scanning ion occlusion sensing. Pharm. Res. 2012, 29, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Kanasova, M.; Nesmerak, K. Systematic review of liposomes’ characterization methods. Monatshefte Chem. Chem. Mon. 2017, 148, 1581–1593. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, S.; Wang, S.; Zhang, W.; Cheng, Y.; Yan, X. Multiparameter Quantification of Liposomal Nanomedicines at the Single-Particle Level by High-Sensitivity Flow Cytometry. ACS Appl. Mater. Interfaces 2017, 9, 13913–13919. [Google Scholar] [CrossRef] [PubMed]
- Saveyn, H.; De Baets, B.; Thas, O.; Hole, P.; Smith, J.; Van Der Meeren, P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J. Colloid Interface Sci. 2010, 352, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Zorin, V.; Siupa, A.; D’Hallewin, M.A.; Guillemin, F.; Bezdetnaya, L. Interaction of liposomal formulations of meta–tetra (hydroxyphenyl) chlorin (Temoporfin) with serum proteins: Protein Binding and Liposome Destruction. Photochem. Photobiol. 2012, 88, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- De Morais Ribeiro, L.N.; Couto, V.M.; Fraceto, L.F.; de Paula, E. Use of nanoparticle concentration as a tool to understand the structural properties of colloids. Sci. Rep. 2018, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Gioria, S.; Caputo, F.; Urban, P.; Maguire, C.M.; Bremer-Hoffmann, S.; Prina-Mello, A.; Calzolai, L.; Mehn, D. Are existing standard methods suitable for the evaluation of nanomedicines: Some case studies. Nanomedicine 2018, 13, 539–554. [Google Scholar] [CrossRef] [PubMed]



| Route of Administration/Dosage Form | Particle Size Range |
|---|---|
| Lymphatic (RES) * | 10–50 nm |
| Long-circulating carriers (brain, tumour) | 50–200 nm |
| Transdermal | 10–600 nm |
| Intravenous/intramuscular | 200–2000 nm |
| Ocular | 100–3000 nm |
| Aerosol | 1–10 µm |
| Nasal | 8–20 µm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. https://doi.org/10.3390/pharmaceutics10020057
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics. 2018; 10(2):57. https://doi.org/10.3390/pharmaceutics10020057
Chicago/Turabian StyleDanaei, M., M. Dehghankhold, S. Ataei, F. Hasanzadeh Davarani, R. Javanmard, A. Dokhani, S. Khorasani, and M. R. Mozafari. 2018. "Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems" Pharmaceutics 10, no. 2: 57. https://doi.org/10.3390/pharmaceutics10020057
APA StyleDanaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh Davarani, F., Javanmard, R., Dokhani, A., Khorasani, S., & Mozafari, M. R. (2018). Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics, 10(2), 57. https://doi.org/10.3390/pharmaceutics10020057