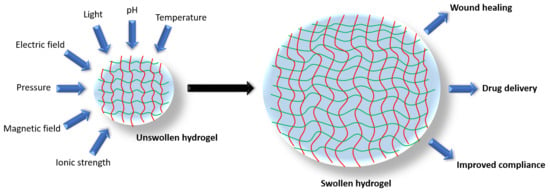
Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle
Abstract
:1. Introduction
2. A Brief Research History of Hydrogels
3. Hydrogel Classification
3.1. Natural Hydrogels
3.2. Synthetic Hydrogels
3.3. Hybrid Hydrogels
4. The Properties of Hydrogels
4.1. Physical Properties
4.2. Mechanical Properties
5. Hydrogels and Wound Healing
6. Hydrogels in Drug Delivery
7. Comparing Hydrogels with Other Drug-Delivery Vehicles
8. Conclusions
Funding
Conflicts of Interest
References
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.G.; Unverdorben, M. Wound cleaning and wound healing: A concise review. Adv. Skin Wound Care 2013, 26, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Monaghan, J.R.; Voss, S.R.; Maden, M. Skin regeneration in adult axolotls: A blueprint for scar-free healing in vertebrates. PLoS ONE 2012, 7, e32875. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Stansby, G.; Avital, L.; Jones, K.; Marsden, G.; Guideline Development Group. Prevention and management of pressure ulcers in primary and secondary care: Summary of NICE guidance. BMJ 2014, 348, g2592. [Google Scholar] [CrossRef] [PubMed]
- Mattera, E.; Iovene, M.R.; Rispoli, C.; Falco, G.; Rocco, N.; Accurso, A. Assessment of bacterial infection in chronic wounds in the elderly: Biopsy versus VERSAJET. Int. J. Surg. 2014, 12 (Suppl. 2), S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Antony, J.; Vafaei, A.; Khan, P.A.; Harrington, A.; Cogo, E.; Wilson, C.; Perrier, L.; Hui, W.; Straus, S.E. Seeking effective interventions to treat complex wounds: An overview of systematic reviews. BMC Med. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Graves, N.; Zheng, H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2014, 22, 20–33. [Google Scholar]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopecek, J. Polymer chemistry: Swell gels. Nature 2002, 417, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for Delivery of Bioactive Agents: A Historical Perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Yahia, L.; Chirani, N.; Gritsch, L.; Motta, F.L.; SoumiaChirani; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 13. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajud, S.A.; Nagda, D.A.; Qiao, P.; Tanaka, N.; Civantos, A.; Gu, R.; Cheng, Z.; Tsourkas, A.; O’Malley, B.W., Jr.; Li, D. A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otol. Neurotol. 2015, 36, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, J.; Truong, N.F.; Segura, T. Design of Cell-Matrix Interactions in Hyaluronic Acid Hydrogel Scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Acuña, R.; García, A.J. Synthetic Hydrogels Mimicking Basement Membrane Matrices to Promote Cell-Matrix Interactions. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Desai, T.; Ferrari, M. Proteins and cells on PEG immobilized silicon surfaces. Biomaterials 1998, 19, 953–960. [Google Scholar] [CrossRef]
- Krsko, P.; Kaplan, J.B.; Libera, M. Spatially controlled bacterial adhesion using surface-patterned poly(ethylene glycol) hydrogels. Acta Biomater. 2009, 5, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Chu, T.-W.; Feng, J.; Yang, J.; Kopeček, J. Hybrid Polymeric Hydrogels via Peptide Nucleic Acid (PNA)/DNA Complexation. J. Control. Release 2015, 220, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.N.; Rao, N.; Christman, K.L. Myocardial Matrix-Polyethylene Glycol Hybrid Hydrogels for Tissue Engineering. Nanotechnology 2014, 25, 014011. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; An, D.; Pardo, Y.; Chiu, A.; Song, W.; Liu, Q.; Zhou, F.; McDonough, S.P.; Ma, M. High-water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta Biomater. 2017, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Rane, A.A.; Christman, K.L. Antibacterial and Cell-adhesive Polypeptide and Poly(ethylene glycol) Hydrogel as a Potential Scaffold for Wound Healing. Acta Biomater. 2012, 8, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.C.; Tomblyn, S.; Burmeister, D.M.; Wrice, N.L.; Becerra, S.C.; Burnett, L.R.; Saul, J.M.; Christy, R.J. Ciprofloxacin-Loaded Keratin Hydrogels Prevent Pseudomonas aeruginosa Infection and Support Healing in a Porcine Full-Thickness Excisional Wound. Adv. Wound Care (New Rochelle) 2015, 4, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, L.E.; West, J.L. Hydrogel-Coated Near Infrared Absorbing Nanoshells as Light-Responsive Drug Delivery Vehicles. ACS Biomater. Sci. Eng. 2015, 1, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, J.; Zhang, M.; Chen, Y.; Wang, X.; Zhang, L.; Tian, Z.; Yan, Y.; Li, Q.; Zhong, W.; et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci. Rep. 2015, 5, 18104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.; Cao, S.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P.; et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Abdel-Raheem, I.T. Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol-chitosan hydrogel containing honey bee venom in diabetic rats. Arch. Pharm. Res. 2014, 37, 1016–1031. [Google Scholar] [CrossRef] [PubMed]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.Y.; Salgado, G.; Lane, E.B.; Hauser, C.A.E. Transparent crosslinked ultrashort peptide hydrogel dressing with high shape-fidelity accelerates healing of full-thickness excision wounds. Sci. Rep. 2016, 6, 32670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Liu, J.-G.; Chen, W.-M.; Yu, A.-X. Efficacy of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with β-cyclodextrin-curcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018, 15, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.W.; Singh, S.P.; Krijgh, D.D.; Yamamoto, M.; Rafii, D.C.; Sung, J.J.; Rafii, S.; Rabbany, S.Y.; Spector, J.A. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regen. 2011, 19, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Yasasvini, S.; Anusa, R.S.; VedhaHari, B.N.; Prabhu, P.C.; RamyaDevi, D. Topical hydrogel matrix loaded with Simvastatin microparticles for enhanced wound healing activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.N.; Martinez-Gonzalez, J.; Chakraborty, I.; Mascharak, P.K. Incorporation of a Theranostic “Two-Tone” Luminescent Silver Complex into Biocompatible Agar Hydrogel Composite for the Eradication of ESKAPE Pathogens in a Skin and Soft Tissue Infection Model. Inorg. Chem. 2018, 57, 6692–6701. [Google Scholar] [CrossRef] [PubMed]
- Laçin, N.T. Development of biodegradable antibacterial cellulose-based hydrogel membranes for wound healing. Int. J. Biol. Macromol. 2014, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Kao, B.; Kadomatsu, K.; Hosaka, Y. Construction of synthetic dermis and skin based on a self-assembled peptide hydrogel scaffold. Tissue Eng. Part A 2009, 15, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Sen, A.; Bae, S.; Lee, J.S.; Webb, K. Poly(ethylene glycol) diacrylate/hyaluronic acid semi-interpenetrating network compositions for 3-D cell spreading and migration. Acta Biomater. 2015, 14, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.V.; Skardal, A.; Song, L.; Sutton, K.; Haug, R.; Mack, D.L.; Jackson, J.; Soker, S.; Atala, A. Solubilized Amnion Membrane Hyaluronic Acid Hydrogel Accelerates Full-Thickness Wound Healing. Stem Cells Transl. Med. 2017, 6, 2020–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocke, N.A.; Arnold, S.M.; Hilt, J.Z. Responsive Hydrogel Nanoparticles for Pulmonary Delivery. J. Drug Deliv. Sci. Technol. 2015, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Figueiras, A.; Veiga, F. Modular Hydrogels for Drug Delivery. J. Biomater. Nanobiotechnol. 2012, 3, 185–199. [Google Scholar] [CrossRef]
- Polo Fonseca, L.; Trinca, R.B.; Felisberti, M.I. Amphiphilic polyurethane hydrogels as smart carriers for acidic hydrophobic drugs. Int. J. Pharm. 2018, 546, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.J.; Thulasidasan, A.K.T.; Anto, R.J.; Chithralekha, D.N.; Narayanan, A.; Kumar, G.S.V. Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J. Nanobiotechnol. 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, G.; Thulasidasan, A.K.T.; Anto, R.J.; Pillai, J.J.; Kumar, G.S.V. Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 2012, 7, 4077–4088. [Google Scholar]
- Carafa, M.; Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Meo, C.; Matricardi, P.; Alhaique, F.; Coviello, T. A new vesicle-loaded hydrogel system suitable for topical applications: Preparation and characterization. J. Pharm. Pharm. Sci. 2011, 14, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Moran, C.A.; Zavgorodnya, O.; Penman, A.D.; Kharlampieva, E.; Bridges, S.L.; Hergenrother, R.W.; Singh, J.A.; Wick, T.M. Development of gellan gum containing formulations for transdermal drug delivery: Component evaluation and controlled drug release using temperature responsive nanogels. Int. J. Pharm. 2016, 509, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Huang, J.J.; Lin, F.C.; Lai, J.Y. Composite poly(2-hydroxyethyl methacrylate) membranes as rate-controlling barriers for transdermal applications. Biomaterials 1997, 18, 527–533. [Google Scholar] [CrossRef]
- Gayet, J.C.; Fortier, G. Drug release from new bioartificial hydrogel. Artif. Cells Blood Substit. Immobil. Biotechnol. 1995, 23, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Mugnier, T.; Courthion, H.; Kranidioti, K.; Karagianni, N.; Denis, M.C.; Lapteva, M.; Kalia, Y.; Möller, M.; Gurny, R. Improved topical delivery of tacrolimus: A novel composite hydrogel formulation for the treatment of psoriasis. J. Control. Release 2016, 242, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Baboota, S.; Alam, M.S.; Sharma, S.; Sahni, J.K.; Kumar, A.; Ali, J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int. J. Pharm. Investig. 2011, 1, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kwankaew, J.; Phimnuan, P.; Wanauppathamkul, S.; Viyoch, J. Formulation of chitosan patch incorporating Artocarpus altilis heartwood extract for improving hyperpigmentation. J. Cosmet. Sci. 2017, 68, 257–269. [Google Scholar] [PubMed]
- Cutting, K.F.; White, R.J. Maceration of the skin and wound bed 1: Its nature and causes. J. Wound Care 2002, 11, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Hydrogel barrier/repair creams and contact dermatitis. Am. J. Contact Dermat. 2000, 11, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Sabale, V.; Vora, S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int. J. Pharm. Investig. 2012, 2, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Trookman, N.; Rizer, R.; Ford, R.; Gotz, V. The importance of vehicle properties to atopic dermatitis patients: A preference study with a novel desonide hydrogel treatment. J. Am. Acad. Dermatol. 2008, 58, AB52. [Google Scholar] [CrossRef]
- Trookman, N.S.; Rizer, R.L. Randomized Controlled Trial of Desonlde Hydrogel 0.05% versus Desonide Ointment 0.05% in the Treatment of Mild-to-moderate Atopic Dermatitis. J. Clin. Aesthet. Dermatol. 2011, 4, 34–38. [Google Scholar] [PubMed]
- Yentzer, B.; Camacho, F.; Young, T.; Fountain, J.; Clark, A.; Feldman, S. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: Results from a program addressing patient compliance. J. Drugs Dermatol. 2010, 9, 324–329. [Google Scholar] [PubMed]
- Kircik, L. Transepidermal Water Loss (TEWL) and Corneometry with Hydrogel Vehicle in the Treatment of Atopic Dermatitis: A Randomized, Investigator-Blind Pilot Study. J. Drugs Dermatol. 2012, 11, 181–184. [Google Scholar]
- Greive, K.A.; Barnes, T.M. Bioequivalence of 0.1% mometasone furoate lotion to 0.1% mometasone furoate hydrogel. Australas. J. Dermatol. 2016, 57, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.; Del Rosso, J. A novel hydrogel vehicle formulated for the treatment of atopic dermatitis. J. Drugs Dermatol. 2007, 6, 718–722. [Google Scholar] [PubMed]
- Kerney, D.L.; Ford, R.O.; Gotz, V. Self-reported participant experience with desonide hydrogel in the treatment of mild to moderate atopic dermatitis. Cutis 2011, 88, 18–24. [Google Scholar] [PubMed]
- Turpeinen, M. Absorption of hydrocortisone from the skin reservoir in atopic dermatitis. Br. J. Dermatol. 1991, 124, 358–360. [Google Scholar] [CrossRef] [PubMed]


| Vehicle | Advantages | Disadvantages |
|---|---|---|
| Creams and lotions |
|
|
| Ointments |
|
|
| Hydrogels |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. https://doi.org/10.3390/pharmaceutics10020071
Harrison IP, Spada F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics. 2018; 10(2):71. https://doi.org/10.3390/pharmaceutics10020071
Chicago/Turabian StyleHarrison, Ian P., and Fabrizio Spada. 2018. "Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle" Pharmaceutics 10, no. 2: 71. https://doi.org/10.3390/pharmaceutics10020071
APA StyleHarrison, I. P., & Spada, F. (2018). Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics, 10(2), 71. https://doi.org/10.3390/pharmaceutics10020071