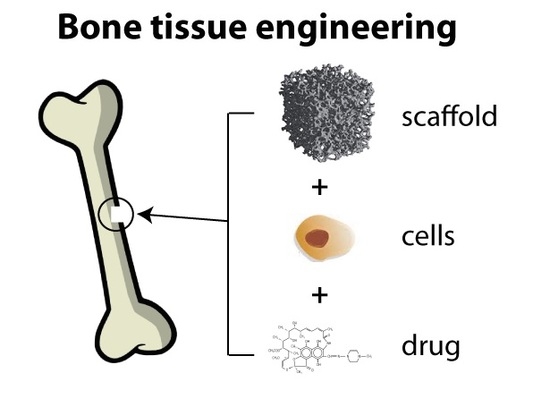
Scaffolds as Structural Tools for Bone-Targeted Drug Delivery
Abstract
:1. Introduction
2. Biomaterials for Bone Scaffolds
2.1. Organic Scaffolds
2.1.1. Synthetic Polymers
2.1.2. Natural Polymers
2.1.3. Lipid Nanoparticles
2.1.4. Purified Bone Allografts
2.2. Inorganic Scaffolds
2.2.1. Metallic Scaffolds
Mesoporous Silica Nanoparticles
Gold Nanoparticles
Nanodiamonds
2.2.2. Ceramic Scaffolds
2.2.3. Composite Xenohybrid Scaffolds
3. New Pharmaceutic Agents in Bone Targeted Therapies
3.1. Growth Factors and PTH
3.2. RNA Interference (RNAi)
3.3. Small Molecules
4. Clinical Applications in Drug Delivery
4.1. Osteomyelitis and Other Orthopaedic Related Infections (ODRIs)
4.2. Cancer Bone Metastasis
4.3. Osteosarcoma and Other Musculoskeletal Malignancies
4.4. Osteoarthritis
4.5. Osteonecrosis
4.6. Pseudo Arthrosis and Delayed-Non Unions
5. Mathematical Modeling
5.1. Modeling Approaches
5.1.1. Microscale Modeling
5.1.2. Macroscale Modeling
- Obstruction theories: polymer chains are considered motionless if compared to solute and solvent molecules. Polymer chains are modeled as fixed impenetrable rods in solution that increase the mean diffusive path of the molecules;
- Hydrodynamic theories: this approach takes into account hydrodynamics interactions, like the frictional ones between the solute and the polymer, the solvent and the polymer and the solute and the solvent;
- Free volume theories: free volume is defined as the volume not occupied by matter or, more generally, as the volume of the system at a given temperature minus the volume of the same system at 0 K. Free volume rearrangements create pores and cavities where diffusing species can diffuse through. In other words, free volume is considered the main factor that determines molecular diffusion.
6. Conclusions
Funding
Conflicts of Interest
Appendix A. On Mathematical Modeling
Appendix A.1. Microscale Modeling
Appendix A.2. Macroscale Modeling: Diffusion
Concerning Diffusion Mechanisms
- Diffusion controlled systems: drug release is determined by the concentration gradient between the loaded device and the external environment. The starting point for the description of such systems is the Fick’s second law:where C is drug concentration in the device, t is time and D is the diffusion coefficient; this parameter depends on several factors (drug, environment, temperature, et cetera) and accounts for the ability of the loaded compound to move within the device. For simple geometries (slabs, cylinders, spheres) and simplified cases (diffusion coefficient constant in time and space, initial uniform drug distribution, and so on) analytical solutions of Equation (A1) are available in literature [196]. The challenge is a robust estimation of the diffusion coefficient in a complex environment such as polymer matrices, as explained below;
- Swelling controlled systems: in systems such as hydrogels, the swelling of the polymer matrix can be the rate determining step for drug release. When the starting point is constituted by a dry (non-swollen) polymer matrix, the drug is embedded in a dense network where the mobility of polymer chains and active compound is strongly hindered. As soon as water starts to penetrate into the matrix, polymer chains relax (their mobility increases) and device volume increases. This dramatically changes the conditions for drug transport from the dry and swollen matrix, since the active molecules become free to move towards the external environment. In this framework, modeling approaches take into account at the same time water penetration into the matrix and drug diffusion, with time and spatial dependent diffusion coefficients that are function of local water concentration. In addition, since matrix volume increases in time because of water penetration, the model has moving boundary conditions that must be properly accounted for [197,198];
- Degradation and erosion controlled systems: as already mentioned, the hydrolysis of the matrix can promote drug release because of the dynamic increase of the diffusion coefficient. Water penetrates into the matrix and starts breaking the backbone of long water-insoluble chains in smaller fragments. In this framework, the meaning of “degradation” and “erosion” must be properly specified. Degradation is the chain scission process, while erosion is the mass loss from the bulk due to the diffusion of the small water-soluble oligomers. The dynamics of water penetration and hydrolysis are determinant. If water penetration is much faster than hydrolysis, water concentration is approximatively uniform into the device volume, which is in turn experiences a uniform degradation; this situation is usually referred as bulk or homogeneous degradation.
Appendix A.3. Macroscale Modeling: Degradation
References
- Carrington, J.L. Aging bone and cartilage: Cross-cutting issues. Biochem. Biophys. Res. Commun. 2005, 328, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, S.J.; Passaro, D.J.; Hershow, R.C.; Layden, J.; Carnes, B.A.; Brody, J.; Hayflick, L.; Butler, R.N.; Allison, D.B.; Ludwig, D.S. A potential decline in life expectancy in the united states in the 21st century. N. Engl. J. Med. 2005, 352, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the ria: A systematic review. Injury 2011, 42 (Suppl. 2), S3–S15. [Google Scholar] [CrossRef]
- Shafiei, Z.; Bigham, A.S.; Dehghani, S.N.; Nezhad, S.T. Fresh cortical autograft versus fresh cortical allograft effects on experimental bone healing in rabbits: Radiological, histopathological and biomechanical evaluation. Cell Tissue Bank. 2009, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Barsa, P.; Buchvald, P.; Svobodnik, A.; Vanickova, E. Autologous versus allogenic bone grafts in instrumented anterior discectomy and fusion: A perspective with respect to bone union pattern. Eur. J. Spine 2004, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, H.; Li, H.; Dai, K.; Wang, J.; Zhang, X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.R.; Nasseri, B.A.; Vacanti, J.P. Tissue engineering: A 21st century solution to surgical reconstruction. Ann. Thorac. Surg. 2001, 72, 577–591. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Ishaug, S.L.; Yaszemski, M.J.; Bizios, R.; Mikos, A.G. Osteoblast function on synthetic biodegradable polymers. J. Biomed. Mater. Res. 1994, 28, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. A 2015, 103, 2482–2498. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for pla-pga biodegradable polymers. Arthroscopy 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Yan, J.; Li, J.; Runge, M.B.; Dadsetan, M.; Chen, Q.; Lu, L.; Yaszemski, M.J. Cross-linking characteristics and mechanical properties of an injectable biomaterial composed of polypropylene fumarate and polycaprolactone co-polymer. J. Biomater. Sci. Polym. Ed. 2011, 22, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Ataollahi, F.; Sayar, K.; Pramanik, S.; Chong, P.P.; Khalil, A.A.; Kamarul, T.; Pingguan-Murphy, B. Chondrogenic potential of physically treated bovine cartilage matrix derived porous scaffolds on human dermal fibroblast cells. J. Biomed. Mater. Res. A 2016, 104, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Li, J.T.; Shoukry, M.; Zhang, Y. A review of decellularized stem cell matrix: A novel cell expansion system for cartilage tissue engineering. Eur. Cell Mater. 2011, 22, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, P.K.; Chandrasekharan, M.; Shyan, J.Y. Recent advances and current developments in tissue scaffolding. Biomed. Mater. Eng. 2005, 15, 159–177. [Google Scholar] [PubMed]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, K.M.; Liu, C. Bone regeneration using photocrosslinked hydrogel incorporating rhbmp-2 loaded 2-n, 6-o-sulfated chitosan nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of nanomaterials for bone-targeted drug delivery. Drug Discov. Today 2017, 22, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Gaudin-Audrain, C.; Mabilleau, G.; Massin, P.; Hubert, L.; Baslè, M.F.; Chappard, D. The influence of processes for the purification of human bone allografts on the matrix surface and cytocompatibility. Biomaterials 2006, 27, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Winckler, H.; Haiden, P. Allograft bone as antibiotic carrier. J. Bone Jt. Infect. 2017, 2, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Kim, J.C.; Kyung, T.W.; Kim, H.J.; Kim, Y.Y.; Kim, S.H.; Kim, J.S.; Choi, H.S. Gold nanoparticles inhibited the receptor activator of nuclear factor-kappab ligand (rankl)-induced osteoclast formation by acting as an antioxidant. Biosci. Biotechnol. Biochem. 2010, 74, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 mapk pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent plla-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Biltz, R.M.; Pellegrino, E.D. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. J. Bone Jt. Surg. Am. 1969, 51, 456–466. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Ambre, A.H.; Katti, D.R.; Katti, K.S. Biomineralized hydroxyapatite nanoclay composite scaffolds with polycaprolactone for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. A 2015, 103, 2077–2101. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3d printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Alves Cardoso, D.; Jansen, J.A.; Leeuwenburgh, S.C. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J. Biomed. Mater. Res. B 2012, 100, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-dbm, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2010, 16, 24–31. [Google Scholar]
- Datta, A.; Gheduzzi, S.; Miles, A.W. A comparison of the viscoelastic properties of bone grafts. Clin. Biomech. 2006, 21, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Capanna, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Knofler, W.; Barth, T.; Graul, R.; Krampe, D. Retrospective analysis of 10,000 implants from insertion up to 20 years-analysis of implantations using augmentative procedures. Int. J. Implant Dent. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; Gabriella, M.; De Angelis, C.; Marco Lupi, S.; Rodriguez y Baena, R. Emerging perspectives in scaffold for tissue engineering in oral surgery. Stem. Cells Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Colaço, H.B.; Shah, Z.; Back, D.; Davies, A.; Ajuied, A. Xenograft in orthopaedics. Orthop. Trauma 2015, 29, 253–260. [Google Scholar] [CrossRef]
- Pertici, G.; Rossi, F.; Casalini, T.; Perale, G. Composite polymer-coated mineral grafts for bone regeneration: Material characterisation and model study. Ann. Oral Maxillofac. Surg. 2014, 2, 4. [Google Scholar]
- Stacchi, C.; Lombardi, T.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral. Implants Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Santoro, M.; Perale, G. Polymeric scaffolds as stem cell carriers in bone repair. J. Tissue Eng. Regen. Med. 2015, 9, 1093–1119. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Perale, G.; Milazzo, M.; Moscato, S.; Stefanini, C.; Pertici, G.; Danti, S. Bovine bone matrix/poly(l-lactic-co-e-caprolactone)/gelatin hybrid scaffold (smartbone1) for maxillary sinus augmentation: A histologic study on bone regeneration. Int. J. Pharm. 2017, 523, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-derived stromal vascular fraction/xenohybrid bone scaffold: An alternative source for bone regeneration. Stem. Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Mbalaviele, G.; Sheikh, S.; Stains, J.P.; Salazar, V.S.; Cheng, S.L.; Chen, D.; Civitelli, R. Beta-catenin and bmp-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell Biochem. 2005, 94, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Vahle, J.L.; Sato, M.; Long, G.G.; Young, J.K.; Francis, P.C.; Engelhardt, J.A.; Westmore, M.S.; Linda, Y.; Nold, J.B. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol. Pathol. 2002, 30, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Pilitsis, J.G.; Lucas, D.R.; Rengachary, S.S. Bone healing and spinal fusion. Neurosurg. Focus 2002, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hosain, F.; Spencer, R.P.; Couthon, H.M.; Sturtz, G.L. Targeted delivery of antineoplastic agent to bone: Biodistribution studies of technetium-99m-labeled gem-bisphosphonate conjugate of methotrexate. J. Nucl. Med. 1996, 37, 105–107. [Google Scholar] [PubMed]
- Lo, K.W.; Ashe, K.M.; Kan, H.M.; Laurencin, C.T. The role of small molecules in musculoskeletal regeneration. Regen. Med. 2012, 7, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, M.D.; Benoit, D.S. Agonism of wnt-beta-catenin signalling promotes mesenchymal stem cell (msc) expansion. J. Tissue Eng. Regen. Med. 2015, 9, E13–E26. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Nuttelman, C.R.; Collins, S.D.; Anseth, K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hmsc differentiation and function for bone regeneration. Biomaterials 2006, 27, 6102–6110. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Ito, H.; Yoshitomi, H.; Yamamoto, K.; Fukuda, A.; Yoshikawa, J.; Furu, M.; Ishikawa, M.; Shibuya, H.; Matsuda, S. Inhibition of mir-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J. Bone Miner. Res. 2014, 29, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S.W. Controlled and sustained delivery of sirna/nps from hydrogels expedites bone fracture healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Jt. Surg. Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef]
- Ripamonti, U.; Reddi, A.H. Growth and morphogenetic factors in bone induction: Role of osteogenin and related bone morphogenetic proteins in craniofacial and periodontal bone repair. Crit. Rev. Oral. Biol. Med. 1992, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ratko, T.A.; Belinson, S.E.; Samson, D.J.; Bonnell, C.; Ziegler, K.M.; Aronson, N. Bone Morphogenetic Protein: The State of the Evidence of on-Label and Off-Label Use; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2010.
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in bmp delivery. Biotechnol. Lett. 2009, 31, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Bucholz, R.W.; Bosse, M.J.; Mirza, S.K.; Lyon, T.R.; Webb, L.X.; Pollak, A.N.; Golden, J.D.; Valentin-Opran, A. Recombinant human bmp-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J. Bone Jt. Surg. Am. 2006, 88, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Emara, K.M.; Diab, R.A.; Emara, A.K. Recent biological trends in management of fracture non-union. World J. Orthop. 2015, 6, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Boraiah, S.; Paul, O.; Hawkes, D.; Wickham, M.; Lorich, D.G. Complications of recombinant human bmp-2 for treating complex tibial plateau fractures: A preliminary report. Clin. Orthop. Relat. Res. 2009, 467, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Shim, W.S.; Kim, S.E.; Lee, K.H.; Kang, E.; Kim, J.H.; Kim, K.; Kwon, I.C.; Lee, D.S. Injectable in situ-forming ph/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng. Part A 2009, 15, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Ejersted, C.; Oxlund, H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J. Bone Miner. Res. 1999, 14, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Cochran, D.L.; Domken, O.; Seibl, R.; Jones, A.A.; Buser, D.; Hammerle, C.H. The effect of matrix bound parathyroid hormone on bone regeneration. Clin. Oral. Implants Res. 2007, 18, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Kumar, V.; Jena, R.; Tunga, R.; Tunga, B.S. Modes of degradation and impurity characterization in rhpth (1–34) during stability studies. PDA J. Pharm. Sci. Technol. 2011, 65, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tu, Q.; Bonewald, L.F.; He, X.; Stein, G.; Lian, J.; Chen, J. Effects of mir-335-5p in modulating osteogenic differentiation by specifically downregulating wnt antagonist dkk1. J. Bone Miner. Res. 2011, 26, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Lietman, S.A.; Ding, C.; Cooke, D.W.; Levine, M.A. Reduction in gsalpha induces osteogenic differentiation in human mesenchymal stem cells. Clin. Orthop. Relat. Res. 2005, 434, 231–238. [Google Scholar] [CrossRef]
- Nelson, C.E.; Kim, A.J.; Adolph, E.J.; Gupta, M.K.; Yu, F.; Hocking, K.M.; Davidson, J.M.; Guelcher, S.A.; Duvall, C.L. Tunable delivery of sirna from a biodegradable scaffold to promote angiogenesis in vivo. Adv. Mater. 2014, 26, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for sirna delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Liu, S.; Wong, H.L. Nanotoxicity: A key obstacle to clinical translation of sirna-based nanomedicine. Nanomedicine 2014, 9, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in sirna delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zintchenko, A.; Philipp, A.; Dehshahri, A.; Wagner, E. Simple modifications of branched pei lead to highly efficient sirna carriers with low toxicity. Bioconjug. Chem. 2008, 19, 1448–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.; Dang, P.N.; Alsberg, E. Functionalized, biodegradable hydrogels for control over sustained and localized sirna delivery to incorporated and surrounding cells. Acta Biomater. 2013, 9, 4487–4495. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Namgung, R.; Kim, W.J. Polymers in small-interfering rna delivery. Nucl. Acid Ther. 2011, 21, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Convertine, A.J.; Benoit, D.S.; Duvall, C.L.; Hoffman, A.S.; Stayton, P.S. Development of a novel endosomolytic diblock copolymer for sirna delivery. J. Control Release 2009, 133, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, D.W.; Freeberg, M.A.T.; Wang, Y.; Sims, K.R., Jr.; Awad, H.A.; Benoit, D.S.W. Diblock copolymer hydrophobicity facilitates efficient gene silencing and cytocompatible nanoparticle-mediated sirna delivery to musculoskeletal cell types. Biomacromolecules 2017, 18, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Luo, E.; Feng, G.; Zhu, S.S.; Li, J.H.; Hu, J. Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos. Int. 2010, 21, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Tai, I.C.; Fu, Y.C.; Wang, C.K.; Chang, J.K.; Ho, M.L. Local delivery of controlled-release simvastatin/plga/hap microspheres enhances bone repair. Int. J. Nanomed. 2013, 8, 3895–3904. [Google Scholar]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Ozec, I.; Kilic, E.; Gumus, C.; Goze, F. Effect of local simvastatin application on mandibular defects. J. Craniofac. Surg. 2007, 18, 546–550. [Google Scholar] [PubMed]
- Bradley, J.D.; Cleverly, D.G.; Burns, A.M.; Helm, N.B.; Schmid, M.J.; Marx, D.B.; Cullen, D.M.; Reinhardt, R.A. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. J. Periodontal. Res. 2007, 42, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.C.; Lima, C.E.; Frederico, L.; Lima, R.P.; Anbinder, A.L. The influence of local administration of simvastatin in calvarial bone healing in rats. J. Craniomaxillofac. Surg. 2011, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.; Lee, Y.; Schmid, M.J.; Killpack, B.; Genrich, M.A.; Narayana, N.; Marx, D.B.; Cullen, D.M.; Reinhardt, R.A. Local simvastatin effects on mandibular bone growth and inflammation. J. Periodontol. 2005, 76, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Lauing, K.L.; Sundaramurthy, S.; Nauer, R.K.; Callaci, J.J. Exogenous activation of wnt/beta-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol. Alcohol. 2014, 49, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Macsai, C.E.; Foster, B.K.; Xian, C.J. Roles of wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J. Cell Physiol. 2008, 215, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Bernick, J.; Wang, Y.; Sigal, I.A.; Alman, B.A.; Whyne, C.M.; Nam, D. Parameters for lithium treatment are critical in its enhancement of fracture-healing in rodents. J. Bone Jt. Surg. Am. 2014, 96, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.H.; Onyia, J.E.; Zeng, Q.; Tian, X.; Liu, M.; Halladay, D.L.; Frolik, C.A.; Engler, T.; Wei, T.; Kriauciunas, A.; et al. Orally bioavailable gsk-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J. Bone Miner. Res. 2006, 21, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Sisask, G.; Marsell, R.; Sundgren-Andersson, A.; Larsson, S.; Nilsson, O.; Ljunggren, O.; Jonsson, K.B. Rats treated with azd2858, a gsk3 inhibitor, heal fractures rapidly without endochondral bone formation. Bone 2013, 54, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Newman, M.R.; Ackun-Farmmer, M.; Baranello, M.P.; Sheu, T.J.; Puzas, J.E.; Benoit, D.S.W. Fracture-targeted delivery of beta-catenin agonists via peptide-functionalized nanoparticles augments fracture healing. ACS Nano 2017, 11, 9445–9458. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Hesse, E. Update on bone anabolics in osteoporosis treatment: Rationale, current status, and perspectives. J. Clin. Endocrinol. Metab. 2012, 97, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Stemberger, A.; Haas, N.P.; Raschke, M. A new model of implant-related osteomyelitis in rats. J. Biomed. Mater. Res. B 2003, 67, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Koort, J.K.; Makinen, T.J.; Suokas, E.; Veiranto, M.; Jalava, J.; Knuuti, J.; Tormala, P.; Aro, H.T. Efficacy of ciprofloxacin-releasing bioabsorbable osteoconductive bone defect filler for treatment of experimental osteomyelitis due to staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Post, V.; Erichsen, C.; Hungerer, S.; Buhren, V.; Militz, M.; Richards, R.G.; Moriarty, T.F. Biofilm formation increases treatment failure in staphylococcus epidermidis device-related osteomyelitis of the lower extremity in human patients. J. Orthop. Res. 2016, 34, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shang, B.C.; Tang, H.; Zhou, T.H.; Xu, G.L.; Li, H.L.; Chen, Q.H.; Xu, Y.Q. Nano-hydroxyapatite/chitosan/konjac glucomannan scaffolds loaded with cationic liposomal vancomycin: Preparation, in vitro release and activity against staphylococcus aureus biofilms. J. Biomater. Sci. Polym. Ed. 2011, 22, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.T.; Chen, C.F.; Chu, I.M.; Li, Y.M.; Hsu, W.H.; Hsu, R.W.; Chang, P.J. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 2010, 31, 5227–5236. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Cenni, E.; Fotia, C.; Avnet, S.; Granchi, D.; Castelli, F.; Micieli, D.; Pignatello, R.; Capulli, M.; Rucci, N.; et al. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr. Cancer Drug Targets 2010, 10, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A. Emerging role of bisphosphonates in the clinic—Antitumor activity and prevention of metastasis to bone. Cancer Treat. Rev. 2008, 34, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Liu, X.Z.; Zhang, L.; Chen, L.B.; Shi, X.; Wu, S.J.; Zhao, J.N. Advances in bone-targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma. Orthop. Surg. 2016, 8, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Clezardin, P.; Benzaid, I.; Croucher, P.I. Bisphosphonates in preclinical bone oncology. Bone 2011, 49, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; McCloskey, E.V. Bisphosphonates in oncology. Bone 2011, 49, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.; Miller, K.; Mero, A.; Satchi-Fainaro, R.; Pasut, G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol. Pharm. 2011, 8, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Elazar, V.; Adwan, H.; Bauerle, T.; Rohekar, K.; Golomb, G.; Berger, M.R. Sustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasis. Int. J. Cancer 2010, 126, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Reufsteck, C.; Lifshitz-Shovali, R.; Zepp, M.; Bauerle, T.; Kubler, D.; Golomb, G.; Berger, M.R. Silencing of skeletal metastasis-associated genes impairs migration of breast cancer cells and reduces osteolytic bone lesions. Clin. Exp. Metastasis 2012, 29, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.J.; Khanna, C. Osteosarcoma genetics and epigenetics: Emerging biology and candidate therapies. Crit. Rev. Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, E.; Volpe, J.; Taylor, T.; Nicksy, D.; Mills, D.; Ramachandran, N.; Shaikh, F.; Riss, V.; Grant, R.; Gupta, A. Outpatient high-dose methotrexate for osteosarcoma: It’s safe and feasible, if you want it. J. Pediatr. Hematol. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hao, M.; Du, X.; Chen, K.; Wang, G.; Yang, J. Advances in targeted therapy for osteosarcoma. Discov. Med. 2014, 17, 301–307. [Google Scholar] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. Plga-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Taruc-Uy, R.L.; Lynch, S.A. Diagnosis and treatment of osteoarthritis. Prim. Care 2013, 40, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J. Osteoarthritis: Something is moving. Reumatol. Clin. 2014, 10, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Morgen, M.; Tung, D.; Boras, B.; Miller, W.; Malfait, A.M.; Tortorella, M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm. Res. 2013, 30, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, R.E.; Wilson, D.S.; Singh, A.; Levenston, M.E.; Murthy, N.; Garcia, A.J. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 2012, 33, 7665–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.H.; Shetty, A.A.; Kim, S.J.; Kim, Y.S.; Choi, N.Y.; Kim, N.H. Treatment of osteonecrosis in the knee joint of a rabbit using autologous cultured osteoblasts. J. Surg. Res. 2013, 185, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Daltro, G.; Hernigou, J. Hip osteonecrosis: Stem cells for life or behead and arthroplasty? Int. Orthop. 2018, 42, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Lim, L.P.; Chong, L.Y.; Dovban, A.S.; Chien, L.Y.; Chung, M.C.; Lei, C.; Kao, M.J.; Chen, C.H.; Chiang, H.C.; et al. Pdgf-simvastatin delivery stimulates osteogenesis in heat-induced osteonecrosis. J. Dent. Res. 2012, 91, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Elniel, A.R.; Giannoudis, P.V. Open fractures of the lower extremity: Current management and clinical outcomes. EFORT Open Rev. 2018, 3, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, S.; Trombi, L.; Bottai, V.; Ghilardi, M.; D’Alessandro, D.; Danti, S.; Dell’Osso, G.; Guido, G.; Petrini, M. Use of autologous human mesenchymal stromal cell/fibrin clot constructs in upper limb non-unions: Long-term assessment. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Le Nail, L.R.; Stanovici, J.; Fournier, J.; Splingard, M.; Domenech, J.; Rosset, P. Percutaneous grafting with bone marrow autologous concentrate for open tibia fractures: Analysis of forty three cases and literature review. Int. Orthop. 2014, 38, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; Krishnakumar, G.S.; Gostynska, N.; Kon, E.; Candrian, C.; Filardo, G. The role of three-dimensional scaffolds in treating long bone defects: Evidence from preclinical and clinical literature—A systematic review. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50. [Google Scholar] [CrossRef]
- Peppas, N.A. Historical perspective on advanced drug delivery: How engineering design and mathematical modeling helped the field mature. Adv. Drug Deliv. Rev. 2013, 65, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Reif, M.M.; Hunenberger, P.H.; Oostenbrink, C. New interaction parameters for charged amino acid side chains in the gromos force field. J. Chem. Theory Comput. 2012, 8, 3705–3723. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. Charmm general force field: A force field for drug-like molecules compatible with the charmm all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 56, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14sb: Improving the accuracy of protein side chain and backbone parameters from ff99sb. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Zgarbova, M.; Otyepka, M.; Sponer, J.; Mladek, A.; Banas, P.; Cheatham, T.E.; Jurecka, P. Nucleic acids force field based on reference quantum chemical calculations of glycosidic torsion profiles. J. Chem. Theory Comput. 2011, 7, 2886–2902. [Google Scholar] [CrossRef] [PubMed]
- Dickson, C.J.; Madej, B.D.; Skjevik, A.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The amber lipid force field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; Gonzalez-Outeirino, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. Glycam06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.S.D.; Liljas, L.; van der Spoel, D. Virus capsid dissolution studied by microsecond molecular dynamics simulations. PLoS Comput. Biol. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.C.; Melo, M.C.R.; Schulten, K. Enhanced sampling techniques in molecular dynamics simulations of biological systems. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 872–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.H.; Luijten, E. Systematic coarse-grained modeling of complexation between small interfering rna and polycations. J. Chem. Phys. 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- Antila, H.S.; Harkonen, M.; Sammalkorpi, M. Chemistry specificity of DNA-polycation complex salt response: A simulation study of DNA, polylysine and polyethyleneimine. Phys. Chem. Chem. Phys. 2015, 17, 5279–5289. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Deriu, M.A.; Patrulea, V.; Borchard, G.; Moller, M.; Danani, A. Free energy landscape of sirna-polycation complexation: Elucidating the effect of molecular geometry, polymer flexibility, and charge neutralization. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ziebarth, J.; Wang, Y.M. Molecular dynamics simulations of DNA-polycation complex formation. Biophys. J. 2009, 97, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.B.; Tang, T.; Uludag, H. Molecular dynamics simulations for complexation of DNA with 2 kda pei reveal profound effect of pei architecture on complexation. J. Phys. Chem. B 2012, 116, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Pavan, G.M. Modeling the interaction between dendrimers and nucleic acids: A molecular perspective through hierarchical scales. Chemmedchem 2014, 9, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Pavan, G.M.; Mintzer, M.A.; Simanek, E.E.; Merkel, O.M.; Kissel, T.; Danani, A. Computational insights into the interactions between DNA and sirna with “rigid” and “flexible” triazine dendrimers. Biomacromolecules 2010, 11, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.B.; Pavan, G.M.; Kasimova, M.R.; Rutherford, S.; Danani, A.; Nielsen, H.M.; Foged, C. Elucidating the molecular mechanism of pamam-sirna dendriplex self-assembly: Effect of dendrimer charge density. Int. J. Pharm. 2011, 416, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.; Schulten, K.; Chipot, C. Calculation of lipid-bilayer permeabilities using an average force. J. Chem. Theory Comput. 2014, 10, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Dickson, C.J.; Hornak, V.; Pearlstein, R.A.; Duca, J.S. Structure-kinetic relationships of passive membrane permeation from multiscale modeling. J. Am. Chem. Soc. 2017, 139, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, D.; Panizon, E.; Ferrando, R.; Monticelli, L.; Rossi, G. Calculating the free energy of transfer of small solutes into a model lipid membrane: Comparison between metadynamics and umbrella sampling. J. Chem. Phys. 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.X.; Anderson, B.D. Liposomal drug transport: A molecular perspective from molecular dynamics simulations in lipid bilayers. Adv. Drug Deliv. Rev. 2006, 58, 1357–1378. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; Berendsen, H.J.C. Permeation process of small molecules across lipid membranes studied by molecular dynamics simulations. J. Phys. Chem. 1996, 100, 16729–16738. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The martini force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; Tieleman, D.P. Perspective on the martini model. Chem. Soc. Rev. 2013, 42, 6801–6822. [Google Scholar] [CrossRef] [PubMed]
- Arnarez, C.; Uusitalo, J.J.; Masman, M.F.; Ingolfsson, H.I.; de Jong, D.H.; Melo, M.N.; Periole, X.; de Vries, A.H.; Marrink, S.J. Dry martini, a coarse-grained force field for lipid membrane simblations with implicit solvent. J. Chem. Theory Comput. 2015, 11, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R. Pathways to structure-property relationships of peptide-materials interfaces: Challenges in predicting molecular structures. Acc. Chem. Res. 2017, 50, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Charchar, P.; Christofferson, A.J.; Todorova, N.; Yarovsky, I. Understanding and designing the gold-bio interface: Insights from simulations. Small 2016, 12, 2395–2418. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.A.; Kwansa, A.L.; Peerless, J.S.; Kim, H.S.; Yingling, Y.G. Advances in molecular modeling of nanoparticle nucleic acid interfaces. Bioconjug. Chem. 2017, 28, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, F.; Pedone, A.; Menziani, M.C. Competitive binding of proteins to gold nanoparticles disclosed by molecular dynamics simulations. J. Phys. Chem. C 2015, 119, 22172–22180. [Google Scholar] [CrossRef]
- Barnard, A.S. Challenges in modelling nanoparticles for drug delivery. J. Phys. Condens. Matter 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance. Langmuir 2011, 27, 13144–13153. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.L.; Qi, W.; Tao, W.; Ma, L.Y.; Fu, C.X. The dynamic behaviours of protein bmp-2 on hydroxyapatite nanoparticles. Mol. Simul. 2011, 37, 1097–1104. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems-a review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Castiglione, F.; Salvalaglio, M.; Ferro, M.; Moioli, M.; Mauri, E.; Masi, M.; Mele, A. On the parallelism between the mechanisms behind chromatography and drug delivery: The role of interactions with a stationary phase. Phys. Chem. Chem. Phys. 2017, 19, 11518–11528. [Google Scholar] [CrossRef] [PubMed]
- Masaro, L.; Zhu, X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Dobrynin, A.V. Theory and simulations of charged polymers: From solution properties to polymeric nanomaterials. Curr. Opin. Colloid Interface Sci. 2008, 13, 376–388. [Google Scholar] [CrossRef]
- Amsden, B.; Grotheer, K.; Angl, D. Influence of polymer ionization degree on solute diffusion in polyelectrolyte gels. Macromolecules 2002, 35, 3179–3183. [Google Scholar] [CrossRef]
- Fatin-Rouge, N.; Milon, A.; Buffle, J.; Goulet, R.R.; Tessier, A. Diffusion and partitioning of solutes in agarose hydrogels: The relative influence of electrostatic and specific interactions. J. Phys. Chem. B 2003, 107, 12126–12137. [Google Scholar] [CrossRef]
- Gu, W.Y.; Yao, H.; Vega, A.L.; Flagler, D. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Ann. Biomed. Eng. 2004, 32, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Casalini, T.; Hulsart-Billstrom, G.; Wang, S.J.; Oommen, O.P.; Salvalaglio, M.; Larsson, S.; Hilborn, J.; Varghese, O.P. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (hpmc). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Mathematical modeling of controlled drug delivery. Adv. Drug Deliv. Rev. 2001, 48, 137–138. [Google Scholar] [CrossRef]
- Lauzon, M.A.; Bergeron, E.; Marcos, B.; Faucheux, N. Bone repair: New developments in growth factor delivery systems and their mathematical modeling. J. Control. Release 2012, 162, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Versypt, A.N.F.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable plga microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.C.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T. Bioresorbability of polymers: Chemistry, mechanisms, and modeling. Bioresorbable Polym. Biomed. Appl. 2017, 120, 65–83. [Google Scholar]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Batycky, R.P.; Hanes, J.; Langer, R.; Edwards, D.A. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J. Pharm. Sci. 1997, 86, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Yamashita, M.; Nagashima, M.; Hattori, N.; Endo, T.; Tokiwa, Y. Theoretical prediction of molecular weight on autocatalytic random hydrolysis of aliphatic polyesters. Macromolecules 2000, 33, 6595–6601. [Google Scholar] [CrossRef]
- Arosio, P.; Busini, V.; Perale, G.; Moscatelli, D.; Masi, M. A new model of resorbable device degradation and drug release—Part I: Zero order model. Polym. Int. 2008, 57, 912–920. [Google Scholar] [CrossRef]
- Perale, G.; Arosio, P.; Moscatelli, D.; Barri, V.; Muller, M.; Maccagnan, S.; Masi, M. A new model of resorbable device degradation and drug release: Transient 1-dimension diffusional model. J. Control. Release 2009, 136, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Lazzari, S.; Perale, G.; Masi, M. Mathematical modeling of plga microparticles: From polymer degradation to drug release. Mol. Pharm. 2014, 11, 4036–4048. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Faisant, N.; Benoit, J.P. A new mathematical model quantifying drug release from bioerodible microparticles using monte carlo simulations. Pharm. Res. 2002, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.; Suryanarayanan, V.; Selvaraj, C.; Singh, S.K.; Singh, P. Explicit drug re-positioning: Predicting novel drug-target interactions of the shelved molecules with qm/mm based approaches. Adv. Protein. Chem. Struct. Biol. 2015, 100, 89–112. [Google Scholar] [PubMed]
- Ganesan, A.; Coote, M.L.; Barakat, K. Molecular dynamics-driven drug discovery: Leaping forward with confidence. Drug Dis. Today 2017, 22, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Dis. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Aqvist, J.; Medina, C.; Samuelsson, J.E. New method for predicting binding-affinity in computer-aided drug design. Protein Eng. 1994, 7, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The mm/pbsa and mm/gbsa methods to estimate ligand-binding affinities. Expert Opin. Drug Dis. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Chodera, J.D.; Mobley, D.L.; Shirts, M.R.; Dixon, R.W.; Branson, K.; Pande, V.S. Alchemical free energy methods for drug discovery: Progress and challenges. Curr. Opin. Struct. Biol. 2011, 21, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Nguyen, P.H.; Pham, K.; Huynh, D.; Le, T.B.N.; Wang, H.L.; Ren, P.Y.; Luo, R. Calculating protein-ligand binding affinities with mmpbsa: Method and error analysis. J. Comput. Chem. 2016, 37, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Lustig, S.R.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. 1. Mathematical-modeling. J. Polym. Sci. Part B 1986, 24, 395–408. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Vonmeerwall, E.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. 2. Verification of theoretical-models. J. Polym. Sci. Part B 1986, 24, 409–434. [Google Scholar] [CrossRef]
- Lee, P.I. Modeling of drug release from matrix systems involving moving boundaries: Approximate analytical solutions. Int. J. Pharm. 2011, 418, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sackett, C.K.; Narasimhan, B. Mathematical modeling of polymer erosion: Consequences for drug delivery. Int. J. Pharm. 2011, 418, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Antheunis, H.; van der Meer, J.C.; de Geus, M.; Heise, A.; Koning, C.E. Autocatalytic equation describing the change in molecular weight during hydrolytic degradation of aliphatic polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.N.; Federspiel, W.J.; Little, S.R. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials 2009, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramkrishna, D. Population Balances: Theory and Applications to Particulate Systems in Engineering; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]


| Disease | Therapeutic Agent | Drug Delivery System | Main Outcome |
|---|---|---|---|
| Osteomyelitis | Antibiotics | PMMA, PLGA | Releases high levels of antibiotic at a local administration site. No side effects. |
| Cancer bone metastasis | DXR | PLGA-ALE | Higher or equal efficacy than free DXR in prevention of osteolytic bone metastases and reduction of DXR concentration in healthy tissues. |
| PTX, ALN | PEG | Marked increase in their half-life. Great binding affinity to the bone in vitro. | |
| Osteosarcoma | DXR | PLGA | Enhance DXR antitumoral efficacy compared with free drug. |
| Osteoarthritis | Dextran | Cationic nanoparticles | Increases the retention time, maintaining cartilage structure and composition. |
| IL-1Ra | IL-1Ra-tethered nanoparticles | ||
| Osteonecrosis | Simvastatin | PDLLA, PLGA | Decrease of inflammation. Facilitates osteogenic differentiation and maturation. |
| PDGF | Decrease of inflammation. Cell recruitment, (imitating the early mitogenic stage in wound healing). | ||
| Delayed-non unions | Osteoinductive agents, antibiotics | Composite systems | Promotes fracture healing and decreases risk of secondary osteomyelitis. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. https://doi.org/10.3390/pharmaceutics10030122
Ferracini R, Martínez Herreros I, Russo A, Casalini T, Rossi F, Perale G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics. 2018; 10(3):122. https://doi.org/10.3390/pharmaceutics10030122
Chicago/Turabian StyleFerracini, Riccardo, Isabel Martínez Herreros, Antonio Russo, Tommaso Casalini, Filippo Rossi, and Giuseppe Perale. 2018. "Scaffolds as Structural Tools for Bone-Targeted Drug Delivery" Pharmaceutics 10, no. 3: 122. https://doi.org/10.3390/pharmaceutics10030122
APA StyleFerracini, R., Martínez Herreros, I., Russo, A., Casalini, T., Rossi, F., & Perale, G. (2018). Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics, 10(3), 122. https://doi.org/10.3390/pharmaceutics10030122