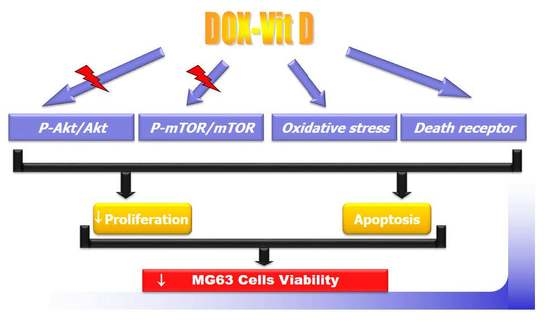
DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemistry
2.2.1. Calciferol-Succinate
2.2.2. Calciferol-Succinate-DOX
2.3. Cell Culture and Treatments
2.4. Effect of DOX and DOX-Vit D on MG63 Cell Proliferation
2.5. RNA Extraction and cDNA Synthesis
2.6. Quantification of mRNA Expression by Quantitative Real-Time Polymerase Chain Reaction (Real Time-PCR)
2.7. Determination of Reactive Oxygen Species (ROS) Production
2.8. Protein Extraction from MG63 Cells
2.9. Immuno Blot Analysis
2.10. Determination of MAPKs Signaling Pathway
2.11. Extration of Nuclear Protein
2.12. Determination of NF-κB Binding Activity
2.13. Statistical Analysis
3. Results
3.1. Physiochemical Properities of DOX-Vit D in Comaprison to DOX
3.2. Effect of DOX and DOX-Vit D on MG63 Cells Proliferation
3.3. Effect of DOX and DOX-Vit D on Proapoptotic Genes
3.4. Effect of DOX and DOX-Vit D on the Expression of DR-4
3.5. Effect of DOX and DOX-Vit D on the Oxidative Stress
3.6. Effect of DOX and DOX-Vit D on MAPK Signaling Pathway
3.7. Effect of DOX and DOX-Vit D on NF-κB Signaling Pathway
3.8. Effect of DOX and DOX-Vit D on Akt and mTOR Signaling Pathway
4. Discussion
Author Contributions
Acknowledgments
Conflict of Interest
References
- Bacci, G.; Longhi, A.; Bertoni, F.; Bacchini, P.; Ruggeri, P.; Versari, M.; Picci, P. Primary high-grade osteosarcoma: Comparison between preadolescent and older patients. J. Pediatr. Hematol. 2005, 27, 129–134. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.E.; Davis, A.M.; Ahn, H.; Wunder, J.S.; Blackstein, M.E.; Bell, R.S. Doxorubicin-cisplatin chemotherapy for high-grade nonosteogenic sarcoma of bone. Comparison of treatment and control groups. Can. J. Surg. 1999, 42, 190–199. [Google Scholar] [PubMed]
- Lewis, I.J.; Nooij, M.A.; Whelan, J.; Sydes, M.R.; Grimer, R.; Hogendoorn, P.C.; Memon, M.A.; Weeden, S.; Uscinska, B.M.; van Glabbeke, M.; et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase iii trial of the european osteosarcoma intergroup. J. Natl. Cancer Inst. 2007, 99, 112–128. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, L. Mechanisms of drug sensitivity and resistance in cancer. Curr. Cancer Drug Targets 2009, 9, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of Vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Epidemiology of disease risks in relation to Vitamin D insufficiency. Prog. Biophys. Mol. Biol. 2006, 92, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between Vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, C.G.; Wilkens, L.R.; Nomura, A.M.; Horst, R.L.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Plasma 25-hydroxyvitamin d levels and the risk of colorectal cancer: The multiethnic cohort study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Eelen, G.; Verlinden, L.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Vitamin D and cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Skinner, H.G. Vitamin D status and cancer: New insights. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Strugnell, S.A.; DeLuca, H.F. Current understanding of the molecular actions of Vitamin D. Physiol. Rev. 1998, 78, 1193–1231. [Google Scholar] [CrossRef] [PubMed]
- Chouvet, C.; Vicard, E.; Devonec, M.; Saez, S. 1,25-dihydroxyvitamin d3 inhibitory effect on the growth of two human breast cancer cell lines (mcf-7, bt-20). J. Steroid Biochem. 1986, 24, 373–376. [Google Scholar] [CrossRef]
- Getzenberg, R.H.; Light, B.W.; Lapco, P.E.; Konety, B.R.; Nangia, A.K.; Acierno, J.S.; Dhir, R.; Shurin, Z.; Day, R.S.; Trump, D.L.; et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the dunning rat prostate model system. Urology 1997, 50, 999–1006. [Google Scholar] [CrossRef]
- Beer, T.M.; Myrthue, A. Calcitriol in cancer treatment: From the lab to the clinic. Mol. Cancer Ther. 2004, 3, 373–381. [Google Scholar] [PubMed]
- Knutson, J.C.; LeVan, L.W.; Valliere, C.R.; Bishop, C.W. Pharmacokinetics and systemic effect on calcium homeostasis of 1 alpha,24-dihydroxyvitamin d2 in rats. Comparison with 1 alpha,25-dihydroxyvitamin d2, calcitriol, and calcipotriol. Biochem. Pharm. 1997, 53, 829–837. [Google Scholar] [CrossRef]
- Wigington, D.P.; Urben, C.M.; Strugnell, S.A.; Knutson, J.C. Combination study of 1,24(s)-dihydroxyvitamin d2 and chemotherapeutic agents on human breast and prostate cancer cell lines. Anticancer Res. 2004, 24, 2905–2912. [Google Scholar] [PubMed]
- Maayah, Z.H.; Althurwi, H.N.; Abdelhamid, G.; Lesyk, G.; Jurasz, P.; El-Kadi, A.O. Cyp1b1 inhibition attenuates doxorubicin-induced cardiotoxicity through a mid-chain hetes-dependent mechanism. Pharm. Res. 2016, 105, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Maayah, Z.H.; El Gendy, M.A.; El-Kadi, A.O.; Korashy, H.M. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome p450 1a1 gene in human breast cancer mcf7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch. Toxicol. 2013, 87, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (mtt) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Maayah, Z.H.; Ansari, M.A.; El Gendy, M.A.; Al-Arifi, M.N.; Korashy, H.M. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Arch. Toxicol. 2014, 88, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Elbekai, R.H.; Korashy, H.M.; Wills, K.; Gharavi, N.; El-Kadi, A.O. Benzo[a]pyrene, 3-methylcholanthrene and beta-naphthoflavone induce oxidative stress in hepatoma hepa 1c1c7 cells by an ahr-dependent pathway. Free Radic. Res. 2004, 38, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Faller, D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991, 19, 2499. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N.; Sarno, A.; Idler, I.S.; Fuhrer, M.; Zenz, T.; Dohner, H.; Stilgenbauer, S.; Mertens, D. High-throughput detection of nuclear factor-kappab activity using a sensitive oligo-based chemiluminescent enzyme-linked immunosorbent assay. Int. J. Cancer 2010, 127, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Alrushaid, S.; Sayre, C.L.; Yanez, J.A.; Forrest, M.L.; Senadheera, S.N.; Burczynski, F.J.; Lobenberg, R.; Davies, N.M. Pharmacokinetic and toxicodynamic characterization of a novel doxorubicin derivative. Pharmaceutics 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Liu, L.Z. Role of mtor in anticancer drug resistance: Perspectives for improved drug treatment. Drug Resist. Updates 2008, 11, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Nuriding, H. Reversal effect of Vitamin D on different multidrug-resistant cells. Genet. Mol. Res. 2014, 13, 6239–6247. [Google Scholar] [CrossRef] [PubMed]
- Sabzichi, M.; Mohammadian, J.; Mohammadi, M.; Jahanfar, F.; Movassagh Pour, A.A.; Hamishehkar, H.; Ostad-Rahimi, A. Vitamin D-loaded nanostructured lipid carrier (nlc): A new strategy for enhancing efficacy of doxorubicin in breast cancer treatment. Nutr. Cancer 2017, 69, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Maessen, P.; van der Vijgh, W.J.; Gall, H.; Boven, E.; Pinedo, H.M. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J. Clin. Oncol. 1988, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Vrignaud, P.; Nguyen-Ngoc, T.; Iliadis, A.; Mauriac, L.; Hurteloup, P. Comparative pharmacokinetics and metabolism of doxorubicin and epirubicin in patients with metastatic breast cancer. Cancer Treat. Rep. 1985, 69, 633–640. [Google Scholar] [PubMed]
- Vecchione, A.; Croce, C.M. Apoptomirs: Small molecules have gained the license to kill. Endoc.-Relat. Cancer 2010, 17, F37–F50. [Google Scholar] [CrossRef] [PubMed]
- Brard, L.; Lange, T.S.; Robison, K.; Kim, K.K.; Ara, T.; McCallum, M.M.; Arnold, L.A.; Moore, R.G.; Singh, R.K. Evaluation of the first ergocalciferol-derived, non hypercalcemic anti-cancer agent mt19c in ovarian cancer skov-3 cell lines. Gynecol. Oncol. 2011, 123, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Huang, Y.T.; Wu, M.L.; Huang, T.C.; Ho, C.T.; Pan, M.H. Induction of apoptosis by Vitamin D2, ergocalciferol, via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia cells. J. Agric. Food Chem. 2008, 56, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Xia, Y.; Guo, C.; Kong, L. Icariin enhances cytotoxicity of doxorubicin in human multidrug-resistant osteosarcoma cells by inhibition of abcb1 and down-regulation of the pi3k/akt pathway. Biol. Pharm. Bull. 2015, 38, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Y.; Zeng, B. Enhanced chemosensitivity by simultaneously inhibiting cell cycle progression and promoting apoptosis of drug-resistant osteosarcoma mg63/dxr cells by targeting cyclin d1 and bcl-2. Cancer Biomark. 2012, 12, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Debatin, K.M. Cellular stress response and apoptosis in cancer therapy. Blood 2001, 98, 2603–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, W.P.; Chau, S.P.; Kong, S.K.; Fung, K.P.; Kwok, T.T. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003, 73, 2047–2058. [Google Scholar] [CrossRef]
- Ravagnan, L.; Roumier, T.; Kroemer, G. Mitochondria, the killer organelles and their weapons. J. Cell. Physiol. 2002, 192, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, J.; Wang, K.; Kong, X.; Liu, H.; Chen, F.; Hu, M.; Zhang, X.; Jiao, X.; Ge, B.; Wu, Y.; et al. Caspase- and p38-mapk-dependent induction of apoptosis in a549 lung cancer cells by newcastle disease virus. Arch. Virol. 2011, 156, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Spitz, D.R.; Holbrook, N.J. Expression of stress response genes gadd153, c-jun, and heme oxygenase-1 in h2o2- and o2-resistant fibroblasts. Free Radic. Biol. Med. 1996, 20, 735–741. [Google Scholar] [CrossRef]
- Mongre, R.K.; Sodhi, S.S.; Ghosh, M.; Kim, J.H.; Kim, N.; Sharma, N.; Jeong, D.K. A new paradigm to mitigate osteosarcoma by regulation of micrornas and suppression of the nf-kappab signaling cascade. Dev. Reprod. 2014, 18, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Garcia-Haro, L.; Sparks, C.A.; Guertin, D.A. Mtor-dependent cell survival mechanisms. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ni, J.; Huang, J. Molecular mechanisms of chemoresistance in osteosarcoma (review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W.; Janeway, K.A. Emerging concepts for pi3k/mtor inhibition as a potential treatment for osteosarcoma. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Pignochino, Y.; Dell’Aglio, C.; Basirico, M.; Capozzi, F.; Soster, M.; Marchio, S.; Bruno, S.; Gammaitoni, L.; Sangiolo, D.; Torchiaro, E.; et al. The combination of sorafenib and everolimus abrogates mtorc1 and mtorc2 upregulation in osteosarcoma preclinical models. Clin. Cancer Res. 2013, 19, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, T.; McSheehy, P.M.; Wartmann, M.; Lassota, P.; Brandt, R.; Lane, H.A. Evaluation of the mtor inhibitor, everolimus, in combination with cytotoxic antitumor agents using human tumor models in vitro and in vivo. Anti-Cancer Drugs 2011, 22, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Congwei, L.; Bei, Q.; Tao, Y.; Ruiguo, W.; Heze, Y.; Bo, D.; Zhihong, L. Mtor: An attractive therapeutic target for osteosarcoma? Oncotarget 2016, 7, 50805–50813. [Google Scholar] [CrossRef] [PubMed]












| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Caspase-3 | GAGTGCTCGCAGCTCATACCT | CCTCACGGCCTGGGATTT |
| P53 | GCCCCCAGGGAGCACTA | GGGAGAGGAGCTGGTGTTG |
| DR4 | AGTACATCTAGGTGCGTTCCTG | GTGCTGTCCCATGGAGGTA |
| BCLxs | CCCAGAAAGGATACAGCTGG | GCGAT-CCGACTCACCAATAC |
| HO-1 | ATGGCCTCCCTGTACCACATC | TGTTGCGCTCAATCTCCTCCT |
| NQO-1 | CGCAGACCTTGTGATATTCCAG | CGTTTCTTCCATCCTTCCAGG |
| β-actin | CCAGATCATGTTTGAGACCTTCAA | GTGGTACGACCAGAGGCATACA |
| Compound | Doxorubicin (Free Base) | Vitamin D2 | DoxVD |
|---|---|---|---|
| Structure |  |  |  |
| Chemical Formula | C27H29NO11 | C28H44O | C59H75NO14 |
| Molecular Weight (g/mol) | 543.53 | 396.65 | 1022.22 |
| LogP (ACD Chemsketch) | 2.82 ± 1.30 | 9.56 ± 0.27 | 12.83 ± 1.32 |
| LogP (VCCLAB) | 1.41 | 7.59 | 5.95 |
| LogP (experimental, Pubchem) | 1.27 | 7.3 | NA |
| Log D7.4 (ACD iLab) | −0.29 | 7.5 | 8.68 |
| Solubility H2O (ACD iLab) | 0.49 mg/mL | 0.0018 mg/mL | 0.0029 μg/mL |
| LogS (VCCLAB) | −2.67 | −5.96 | −5.81 |
| Solubility (experimental, drug bank) | 2% | 0.05 mg/mL | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maayah, Z.H.; Zhang, T.; Forrest, M.L.; Alrushaid, S.; Doschak, M.R.; Davies, N.M.; El-Kadi, A.O.S. DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways. Pharmaceutics 2018, 10, 144. https://doi.org/10.3390/pharmaceutics10030144
Maayah ZH, Zhang T, Forrest ML, Alrushaid S, Doschak MR, Davies NM, El-Kadi AOS. DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways. Pharmaceutics. 2018; 10(3):144. https://doi.org/10.3390/pharmaceutics10030144
Chicago/Turabian StyleMaayah, Zaid H., Ti Zhang, Marcus Laird Forrest, Samaa Alrushaid, Michael R. Doschak, Neal M. Davies, and Ayman O. S. El-Kadi. 2018. "DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways" Pharmaceutics 10, no. 3: 144. https://doi.org/10.3390/pharmaceutics10030144
APA StyleMaayah, Z. H., Zhang, T., Forrest, M. L., Alrushaid, S., Doschak, M. R., Davies, N. M., & El-Kadi, A. O. S. (2018). DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and mTOR Signaling Pathways. Pharmaceutics, 10(3), 144. https://doi.org/10.3390/pharmaceutics10030144