Simultaneous Determination of Chlorogenic Acid Isomers and Metabolites in Rat Plasma Using LC-MS/MS and Its Application to A Pharmacokinetic Study Following Oral Administration of Stauntonia Hexaphylla Leaf Extract (YRA-1909) to Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Clibration Standards and Quality Control Samples
2.3. Sample Preparation
2.4. LC-MS/MS Analysis
2.5. Method Validation
2.6. Pharmacokinetic Study of YRA-1909 in Rats
3. Results
3.1. LC-MS/MS Analysis
3.2. Method Validation
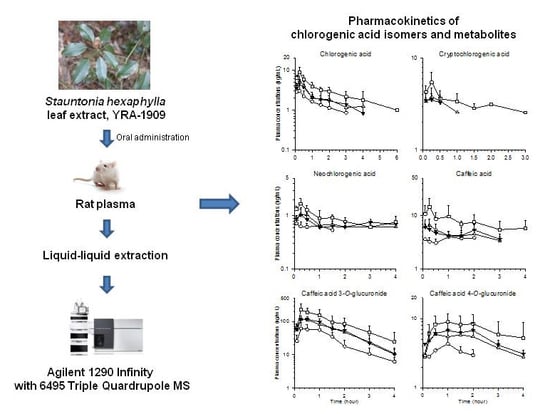
3.3. Pharmacokinetics of YRA-1909 in Male SD Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.-B.; Mayer, R.; Rücker, G.; Yang, J.-J.; Matteson, D.S. A phenolic glycoside and triterpenoids from Stauntonia hexaphylla. Phytochemistry 1998, 47, 467–470. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, Y.S.; Towantakavanit, K.; Park, J.O.; Kim, Y.M.; Jung, K.J.; Cho, J.Y.; Lee, K.D.; Heo, B.G. Chemical components and biological activity of Stauntonia hexaphylla. Korean J. Plant. Resour. 2009, 22, 403–411. [Google Scholar]
- Cheon, Y.H.; Baek, J.M.; Park, S.H.; Ahn, S.J.; Lee, M.S.; Oh, J.; Kim, J.Y. Stauntonia hexaphylla (Lardizabalaceae) leaf methanol extract inhibits osteoclastogenesis and bone resorption activity via proteasome-mediated degradation of c-Fos protein and suppression of NFATc1 expression. BMC Complement. Altern. Med. 2015, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kwon, S.H.; Kim, S.B.; Lim, S.S. Inhibitory activities of Stauntonia hexaphylla leaf constituents on rat lens aldose reductase and formation of advanced glycation end products and antioxidant. Biomed. Res. Int. 2017, 2017, 4273257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Choi, H.; Jo, A.; Kang, H.; Yun, H.; Im, S.; Choi, C. Anti-inflammatory effects of a Stauntonia hexaphylla Fruit Extract in lipopolysaccharide-activated RAW-264.7 macrophages and rats by carrageenan-induced hind paw swelling. Nutrients 2018, 10, E110. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Kim, J.Y.; Kang, S.E.; Yoo, J.S.; Lee, Y.; Lee, D.G.; Park, J.S.; Lee, E.B.; Lee, E.Y.; Song, Y.W. YRA-1909 suppresses production of pro-inflammatory mediators and MMPs through downregulating Akt, p38, JNK and NF-κb activation in rheumatoid arthritis fibroblast-like synoviocytes. In Proceedings of the 81st American College of Rheumatology and the 52nd Association of Rheumatology Health Professionals Annual Scientific Meeting, San Diego, CA, USA, 6 November 2017. Abstract Number, 1412. [Google Scholar]
- A Phase 2 Study to Evaluate the Safety and Efficacy of YRA-1909 in Patients with Rheumatoid Arthritis. Available online: https://clinicaltrials.gov/ct2/archive/NCT03275025 (accessed on 7 November 2017).
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Lseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007, 55, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.; Wang, H.; He, Z.; Ooi, V.E. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2006, 106, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S. Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid. J. Biochem. Mol. Toxicol. 2017, 31, e21865. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stalmach, A.; Calani, L.; Crozier, A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients 2010, 2, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Dew, T.P.; Poquet, L.; Hanson, P.; Williamson, G. Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab. Dispos. 2011, 39, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.; Mullen, W.; Stalmach, A.; Auger, C.; Rouanet, J.M.; Teissedre, P.L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, disposition, metabolism, and excretion of [3-(14)C] caffeic acid in rats. J. Agric. Food Chem. 2012, 60, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Meinl, W.; Glatt, H.R.; Barron, D.; Stalmach, A.; Steiling, H.; Crozier, A.; Williamson, G. In vitro and in vivo conjugation of dietary hydroxycinnamic acids by UDP-glucuronosyltransferases and sulfotransferases in humans. J. Nutr. Biochem. 2010, 21, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Tomita, Y. Antimutagenic activity of caffeic acid and related compounds. Biosci. Biotech. Biochem. 1996, 60, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dou, D.; Ge, L.; Huang, Z.; Wang, L.; Gu, N. A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti-cancer activity. Colloids Surf. B Biointerfaces 2015, 134, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Lin, X.; Yao, H. A comprehensive review of recent studies on pharmacokinetics of traditional Chinese medicines (2014–2017) and perspectives. Drug Metab. Rev. 2018, 50, 161–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Tai, W.; Yang, L.; Chen, Y.; Chen, C.; Liu, C. Challenges and solutions of pharmacokinetics for efficacy and safety of traditional chinese medicine. Curr. Drug Metab. 2015, 16, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, D.K.; Ji, H.Y.; Oh, S.R.; Lee, H.K.; Lee, H.S. Liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry for the simultaneous determination of dimethoxyaschantin, dimethylliroresinol, dimethylpinoresinol, epimagnolin A, fargesin and magnolin in rat plasma. Biomed. Chromatogr. 2011, 25, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.; Li, W.; Chu, Y.; Guo, J.; Li, S.; Wang, J.; Zhang, H.; Zhou, S.; Zhu, Y. Simultaneous determination of five phenolic components and paeoniflorin in rat plasma by liquid chromatography-tandem mass spectrometry and pharmacokinetic study after oral administration of Cerebralcare granule. J. Pharm. Biomed. Anal. 2013, 86, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, S.; Ju, W.; Shan, J.; Meng, M.; Cai, B.; Di, L. Simultaneous determination of phenolic acids by UPLC-MS/MS in rat plasma and its application in pharmacokinetic study after oral administration of Flos Lonicerae preparations. J. Pharm. Biomed. Anal. 2013, 86, 189–197. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.; Pinto, C.B.; Sampaio, G.R.; Yonekura, L.; Catharino, R.R.; Bastos, D.H. Development and validation of methods for the extraction of phenolic acids from plasma, urine, and liver and analysis by UPLC-MS. J. Agric. Food Chem. 2013, 61, 6113–6121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shan, J.; Wang, S.; Ju, W.; Meng, M.; Cai, B.; Di, L. Simultaneous determination of caffeic acid derivatives by UPLC-MS/MS in rat plasma and its application in pharmacokinetic study after oral administration of Flos Lonicerae-Fructus Forsythiae herb combination. J. Chromatogr. B 2014, 949–950, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, M.; Yuan, Y.; Guo, B.; Zhou, J.; Tan, Z.; Ye, M.; Ding, L.; Chen, B. Simultaneous multi-component quantitation of Chinese herbal injection Yin-zhi-huang in rat plasma by using a single-tube extraction procedure for mass spectrometry-based pharmacokinetic measurement. J. Chromatogr B 2014, 967, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Marmet, C.; Actis-Goretta, L.; Renouf, M.; Giuffrida, F. Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC-MS/MS after oral ingestion of soluble coffee. J. Pharm. Biomed. Anal. 2014, 88, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Ma, X.; Chu, Y.; Li, S.; Guo, J.; Jia, Y.; Zhou, S.; Zhu, Y.; Liu, C. Simultaneous determination of caffeic acid and its major pharmacologically active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Ge, A.H.; Yu, X.A.; Jiao, X.C.; Li, J.; He, J.; Tian, J.; Liu, W.; Azietaku, J.T.; Zhang, B.L.; et al. Simultaneous determination of four phenolic acids and seven alkaloids in rat plasma after oral administration of traditional Chinese medicinal preparation Jinqi Jiangtang Tablet by LC-ESI-MS/MS. J. Pharm. Biomed. Anal. 2016, 117, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, J.M.; Jeong, J.S.; Son, M.; Lee, H.S.; Lee, M.G.; Kang, H.E. Pharmacokinetics of chlorogenic acid and corydaline in DA-9701, a new botanical gastroprokinetic agent, in rats. Xenobiotica 2014, 44, 635–643. [Google Scholar] [CrossRef] [PubMed]




| Analytes | Concentration Range (ng/mL), Linear Equation a, Linearity (r2) b, LLOQ (ng/mL) | QC Concentration (ng/mL) | Intra-day (n = 5) | Inter-day (n = 15) | ||
|---|---|---|---|---|---|---|
| RE (%) | CV (%) | RE (%) | CV (%) | |||
| CGA | 0.5–200 | 0.5 | 12.0 | 8.9 | 2.0 | 11.8 |
| y = 0.01110x − 0.00054 | 1.5 | −6.7 | 6.4 | −4.7 | 11.2 | |
| 0.9981 | 25 | 2.0 | 3.7 | −1.9 | 10.5 | |
| 0.5 | 160 | 10.2 | 2.4 | 0.1 | 11.8 | |
| NCGA | 0.5–200 | 0.5 | −2.0 | 4.1 | −2.0 | 8.2 |
| y = 0.00330x − 0.00039 | 1.5 | −10.0 | 3.0 | −1.3 | 10.1 | |
| 0.9983 | 25 | 0.7 | 3.1 | 2.8 | 8.0 | |
| 0.5 | 160 | 10.4 | 2.0 | 2.0 | 12.5 | |
| CCGA | 2.5–1000 | 2.5 | 16.0 | 7.2 | 3.6 | 11.6 |
| y = 0.00149x − 0.00068 | 7.5 | −6.3 | 4.8 | −3.9 | 6.7 | |
| 0.9974 | 125 | 3.0 | 4.2 | 2.8 | 5.6 | |
| 2.5 | 800 | −4.4 | 3.2 | −1.9 | 8.3 | |
| CA | 2.5–1000 | 2.5 | −8.8 | 7.9 | −2.8 | 18.1 |
| y = 0.03784x − 0.00235 | 7.5 | −3.9 | 8.3 | −2.8 | 8.6 | |
| 0.9959 | 125 | 6.5 | 5.0 | 6.5 | 6.2 | |
| 2.5 | 800 | 4.1 | 5.8 | 0.9 | 9.2 | |
| CA-3-G | 2.5–1000 | 2.5 | 0.8 | 7.1 | −0.4 | 8.0 |
| y = 0.00174x + 0.00022 | 7.5 | −8.3 | 4.2 | −5.9 | 9.6 | |
| 0.9981 | 125 | 4.3 | 2.4 | 2.4 | 6.2 | |
| 2.5 | 800 | 8.8 | 5.1 | 0.9 | 10.4 | |
| CA-4-G | 2.5–1000 | 2.5 | −6.8 | 10.7 | −5.2 | 9.3 |
| y = 0.00140x + 0.00031 | 7.5 | −7.2 | 7.0 | 0.3 | 11.6 | |
| 0.9977 | 125 | 10.0 | 3.5 | 6.7 | 6.4 | |
| 2.5 | 800 | 7.7 | 4.7 | 1.2 | 9.6 | |
| FA | 12.5–5000 | 12.5 | 12.6 | 8.5 | 9.0 | 7.7 |
| y = 0.00628x − 0.02194 | 37.5 | −3.2 | 7.2 | −6.5 | 7.7 | |
| 0.9969 | 625 | −3.0 | 3.8 | −7.4 | 6.0 | |
| 12.5 | 4000 | 4.8 | 3.9 | −0.1 | 9.6 | |
| Compounds | Nominal Concentration (ng/mL) | Matrix Effect a (%) | Recovery b (Mean ± SD, %) | |
|---|---|---|---|---|
| Mean | CV (%) | |||
| CGA | 1.5 | 91.6 | 7.6 | 63.0 ± 6.3 |
| 25 | 92.0 | 11.0 | 71.7 ± 6.9 | |
| 160 | 87.1 | 5.4 | 71.9 ± 9.4 | |
| NCGA | 1.5 | 96.1 | 3.6 | 41.4 ± 4.0 |
| 25 | 90.8 | 3.2 | 43.4 ± 3.7 | |
| 160 | 89.0 | 7.1 | 41.8 ± 1.4 | |
| CCGA | 7.5 | 98.0 | 6.6 | 61.5 ± 5.2 |
| 125 | 92.4 | 2.2 | 63.0 ± 7.8 | |
| 800 | 89.3 | 6.2 | 65.2 ± 5.7 | |
| CA | 7.5 | 87.7 | 7.2 | 94.0 ± 9.9 |
| 125 | 93.0 | 11.8 | 86.1 ± 10.4 | |
| 800 | 88.6 | 6.2 | 91.2 ± 6.3 | |
| CA-3-G | 7.5 | 95.9 | 2.0 | 46.5 ± 4.3 |
| 125 | 95.8 | 7.4 | 51.2 ± 3.4 | |
| 800 | 88.5 | 8.5 | 52.8 ± 4.2 | |
| CA-4-G | 7.5 | 92.7 | 1.7 | 51.3 ± 6.0 |
| 125 | 95.6 | 5.3 | 53.0 ± 3.4 | |
| 800 | 86.5 | 9.7 | 55.8 ± 4.2 | |
| FA | 37.5 | 96.8 | 2.2 | 93.3 ± 8.7 |
| 625 | 87.5 | 8.4 | 96.9 ± 8.7 | |
| 4000 | 97.5 | 8.6 | 97.4 ± 4.2 | |
| FA-d3 | 20 | 96.0 | 5.4 | 100.1 ± 2.0 |
| Analytes and Nominal Concentration (ng/mL) | Post-Preparative (24 h at 4 °C) | Short-Term (2 h on ice) | Long-Term (28 days at −80 °C) | Freeze-Thaw 3 Cycles (−80 °C to room temp.) | ||||
|---|---|---|---|---|---|---|---|---|
| CV, % | RE, % | CV, % | RE, % | CV, % | RE, % | CV, % | RE, % | |
| CGA | ||||||||
| 1.5 | 8.8 | −9.3 | 2.3 | −12.0 | 7.0 | −14.0 | 6.2 | −14.0 |
| 160 | 2.1 | 9.4 | 2.5 | −5.4 | 4.1 | −12.3 | 3.2 | −10.0 |
| NCGA | ||||||||
| 1.5 | 14.4 | −2.7 | 2.3 | −12.0 | 3.7 | −9.3 | 10.1 | −14.0 |
| 160 | 2.7 | 11.2 | 4.2 | −5.9 | 5.6 | −6.3 | 5.9 | −5.3 |
| CCGA | ||||||||
| 7.5 | 5.1 | −3.2 | 3.7 | −14.3 | 5.9 | −8.9 | 7.6 | −11.9 |
| 800 | 1.3 | 5.5 | 2.9 | −4.6 | 4.4 | −14.4 | 3.0 | −9.4 |
| CA | ||||||||
| 7.5 | 4.1 | −0.1 | 9.0 | −12.4 | 7.3 | −6.3 | 8.3 | −7.2 |
| 800 | 0.9 | 10.6 | 2.1 | −7.7 | 7.6 | −12.7 | 2.1 | −11.0 |
| CA-3-G | ||||||||
| 7.5 | 9.9 | −10.0 | 6.1 | −14.6 | 7.3 | −7.1 | 9.1 | −10.5 |
| 800 | 1.1 | 9.1 | 2.8 | −1.5 | 2.2 | −14.8 | 4.4 | −11.9 |
| CA-4-G | ||||||||
| 7.5 | 6.2 | −9.6 | 8.1 | −9.3 | 7.9 | 1.6 | 4.7 | −3.6 |
| 800 | 1.0 | 10.9 | 3.2 | −0.4 | 2.1 | −13.9 | 4.7 | −9.4 |
| FA | ||||||||
| 37.5 | 3.2 | −7.0 | 5.4 | −11.2 | 5.9 | −11.0 | 5.6 | −7.7 |
| 4000 | 4.6 | −8.6 | 2.7 | −4.8 | 6.1 | −8.8 | 3.9 | −8.4 |
| Compounds | PK Parameters | Dose of YRA-1909 (mg/kg) | |||
|---|---|---|---|---|---|
| Single Oral Dosing | 7-Day Repeated Oral Dosing | ||||
| 25 (n = 6) | 50 (n =12) | 100 (n = 6) | 50 (n = 6) | ||
| CGA | Cmax (ng/mL) | 3.11 ± 0.96 | 5.59 ± 1.61 | 8.73 ± 2.83 | 4.85 ± 1.25 |
| Tmax 1 (h) | 0.25 (0.083–0.25) | 0.25 (0.083–0.5) | 0.25 | 0.25 (0.083–0.5) | |
| AUClast (ng∙h/mL) | 4.29 ± 1.92 | 8.16 ± 3.65 | 14.38 ± 5.06 | 7.54 ± 1.86 | |
| t1/2 (h) | NC | NC | 1.52 ± 0.79 | NC | |
| MRT (h) | 1.19 ± 0.22 | 1.48 ± 0.50 | 1.67 ± 0.63 | 1.48 ± 0.18 | |
| CCGA | Cmax (ng/mL) | NC | 4.01 ± 0.82 | 5.64 ± 1.45 | 3.81 ± 0.72 |
| Tmax 1 (h) | NC | 0.25 (0.083–0.5) | 0.25 (0.083–0.25) | 0.25 (0.083–0.5) | |
| AUClast (ng∙h/mL) | NC | 4.15 ± 4.10 | 6.26 ± 4.70 | 3.35 ± 3.42 | |
| MRT (h) | NC | 0.61 ± 0.55 | 0.84 ± 0.65 | 0.56 ± 0.57 | |
| NCGA | Cmax (ng/mL) | 0.70 ± 0.12 | 1.18 ± 0.26 | 1.69 ± 0.43 | 1.08 ± 0.28 |
| Tmax 1 (h) | 0.17 (0.083–0.5) | 0.25 (0.083–0.5) | 0.25 (0.083–0.25) | 0.25 (0.083–0.25) | |
| AUClast (ng∙h/mL) | 0.81 ± 0.22 | 1.61 ± 0.90 | 3.40 ± 1.97 | 1.60 ± 0.79 | |
| MRT (h) | 0.68 ± 0.30 | 1.00 ± 0.58 | 1.66 ± 0.91 | 1.09 ± 0.55 | |
| CA | Cmax (ng/mL) | 3.92 ± 1.47 | 7.31 ± 2.12 | 16.72 ± 5.45 | 6.07 ± 0.94 |
| Tmax 1 (h) | 1.25 (1.0–1.5) | 0.25 (0.083–1.0) | 0.25 (0.25–2.0) | 0.17 (0.083–2.0) | |
| AUClast (ng∙h/mL) | 5.60 ± 1.30 | 12.40 ± 4.03 | 29.73 ± 15.24 | 11.75 ± 3.31 | |
| MRT (h) | 0.90 ± 0.11 | 1.29 ± 0.31 | 1.74 ± 0.87 | 1.26 ± 0.33 | |
| CA-3-G | Cmax (ng/mL) | 69.09 ± 25.06 | 129.05 ± 30.41 | 238.55 ± 97.46 | 132.18 ± 26.70 |
| Tmax 1 (h) | 0.38 (0.25–1.0) | 0.38 (0.25–1.0) | 0.25 (0.25–1.0) | 0.38 (0.25–1.0) | |
| AUClast (ng∙h/mL) | 111.15 ± 37.32 | 208.77 ± 80.46 | 405.33 ± 188.56 | 216.38 ± 25.06 | |
| t1/2 (h) | 1.05 ± 0.38 | 0.93 ± 0.23 | 0.82 ± 0.23 | 0.88 ± 0.19 | |
| MRT (h) | 1.49 ± 0.52 | 1.33 ± 0.24 | 1.68 ± 0.69 | 1.33 ± 0.12 | |
| CA-4-G | Cmax (ng/mL) | 3.86 ± 0.94 | 6.97 ± 1.91 | 9.38 ± 2.56 | 7.77 ± 1.85 |
| Tmax 1 (h) | 1.0 (1.0–1.5) | 0.75 (0.25–2.0) | 1.0 (0.5–2.0) | 1.5 (1.0–2.0) | |
| AUClast (ng∙h/mL) | 6.04 ± 0.77 | 14.95 ± 4.03 | 25.59 ± 10.84 | 18.95 ± 3.30 | |
| MRT (h) | 1.01 ± 0.18 | 1.48 ± 0.27 | 1.77 ± 0.46 | ± 0.23 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.-G.; Kim, J.-H.; Kim, D.K.; Lee, Y.; Yoo, J.S.; Shin, D.H.; Lee, H.S. Simultaneous Determination of Chlorogenic Acid Isomers and Metabolites in Rat Plasma Using LC-MS/MS and Its Application to A Pharmacokinetic Study Following Oral Administration of Stauntonia Hexaphylla Leaf Extract (YRA-1909) to Rats. Pharmaceutics 2018, 10, 143. https://doi.org/10.3390/pharmaceutics10030143
Choi W-G, Kim J-H, Kim DK, Lee Y, Yoo JS, Shin DH, Lee HS. Simultaneous Determination of Chlorogenic Acid Isomers and Metabolites in Rat Plasma Using LC-MS/MS and Its Application to A Pharmacokinetic Study Following Oral Administration of Stauntonia Hexaphylla Leaf Extract (YRA-1909) to Rats. Pharmaceutics. 2018; 10(3):143. https://doi.org/10.3390/pharmaceutics10030143
Chicago/Turabian StyleChoi, Won-Gu, Ju-Hyun Kim, Dong Kyun Kim, Yongnam Lee, Ji Seok Yoo, Dae Hee Shin, and Hye Suk Lee. 2018. "Simultaneous Determination of Chlorogenic Acid Isomers and Metabolites in Rat Plasma Using LC-MS/MS and Its Application to A Pharmacokinetic Study Following Oral Administration of Stauntonia Hexaphylla Leaf Extract (YRA-1909) to Rats" Pharmaceutics 10, no. 3: 143. https://doi.org/10.3390/pharmaceutics10030143
APA StyleChoi, W. -G., Kim, J. -H., Kim, D. K., Lee, Y., Yoo, J. S., Shin, D. H., & Lee, H. S. (2018). Simultaneous Determination of Chlorogenic Acid Isomers and Metabolites in Rat Plasma Using LC-MS/MS and Its Application to A Pharmacokinetic Study Following Oral Administration of Stauntonia Hexaphylla Leaf Extract (YRA-1909) to Rats. Pharmaceutics, 10(3), 143. https://doi.org/10.3390/pharmaceutics10030143