New ABCC2 rs3740066 and rs2273697 Polymorphisms Identified in a Healthy Colombian Cohort
Abstract
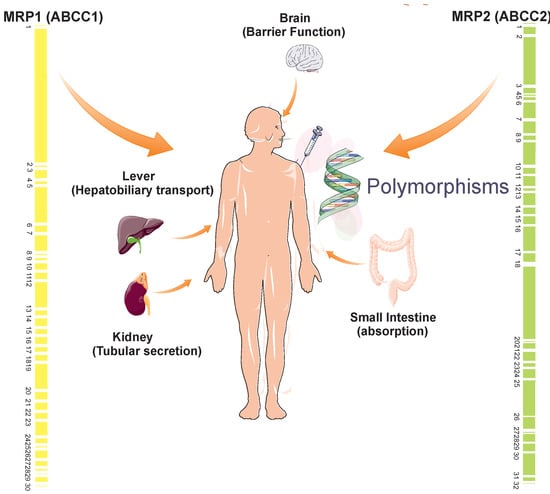
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PCR and DNA Sequencing
2.3. Sequencing Analysis
2.4. Population Analysis of Polymorphisms
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’Graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- NCBI, ABCC2 ATP-Binding Cassette, Sub-Family C (CFTR/MRP), Member 2 [Homo Sapiens (Human)]. Available online: http://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=full_report&list_uids=1244#reference-sequences (accessed on 12 July 2018).
- Choudhuri, S.; Klaassen, C.D. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006, 25, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, J. Multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphism: From discovery to clinical application. Zhong Nan Da Xue Xue Bao Yi Xue Ban (J. Cent. South Univ. Med. Sci.) 2011, 36, 927–938. [Google Scholar]
- Da Costa, K.M.; Valente, R.C.; Salustiano, E.J.; Gentile, L.B.; Freire-de-Lima, L.; Mendonca-Previato, L.; Previato, J.O. Functional Characterization of ABCC Proteins from Trypanosoma cruzi and Their Involvement with Thiol Transport. Front. Microbiol. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Ocsovszki, I.; Pusztai, R. Amyloid-beta Interactions with ABC Transporters and Resistance Modifiers. Anticancer Res. 2018, 38, 3407–3410. [Google Scholar] [CrossRef] [PubMed]
- Tsakalozou, E.; Adane, E.D.; Kuo, K.L.; Daily, A.; Moscow, J.A.; Leggas, M. The effect of breast cancer resistance protein, multidrug resistant protein 1, and organic anion-transporting polypeptide 1B3 on the antitumor efficacy of the lipophilic camptothecin 7-t-butyldimethylsilyl-10-hydroxycamptothecin (AR-67) in vitro. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.E.; Kurz, E.U.; Cole, S.P.; Deeley, R.G. Analysis of the intron-exon organization of the human multidrug-resistance protein gene (MRP) and alternative splicing of its mRNA. Genomics 1997, 45, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Gene Cards, Genomics for ABCC1 Gene. Available online: https://www.genecards.org/ (accessed on 12 July 2018).
- Conrad, S.; Kauffmann, H.M.; Ito, K.; Leslie, E.M.; Deeley, R.G.; Schrenk, D.; Cole, S.P. A naturally occurring mutation in MRP1 results in a selective decrease in organic anion transport and in increased doxorubicin resistance. Pharmacogenetics 2002, 12, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, C.J.; Spitzenberger, T.J.; Elmquist, W.F.; Miller, D.W. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood-brain barrier. Pharm. Res. 2005, 22, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Nutrigene, CFTR/MRP (Subfamily C). Available online: http://nutrigene.4t.com/humanabc.htm#CFTR/MRP (accessed on 12 July 2018).
- Sosnik, A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “Generally Recognized As Safe” (GRAS) nanopharmaceuticals: A review. Adv. Drug Deliv. Rev. 2013, 65, 1828–1851. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Svenningsen, K.; Knudsen, L.A.; Hansen, A.K.; Holmskov, U.; Stensballe, A.; Vogel, U. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J. Gastroenterol. 2015, 21, 11862–11876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: Obstacles or opportunities for drug delivery? Curr. Pharm. Des. 2014, 20, 1422–1449. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ieiri, I.; Tanabe, M.; Suzuki, A.; Higuchi, S.; Otsubo, K. Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics 2001, 11, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Decosterd, L.; Kerb, R.; Telenti, A. Pharmacogenetics in Infectious Diseases. In Pharmacogenetics; Hall, I., Pirmohamed, M., Eds.; Taylos & Francis: London, UK, 2006; pp. 155–178. [Google Scholar]
- Moriya, Y.; Nakamura, T.; Horinouchi, M.; Sakaeda, T.; Tamura, T.; Aoyama, N.; Shirakawa, T.; Gotoh, A.; Fujimoto, S.; Matsuo, M.; et al. Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol. Pharm. Bull. 2002, 25, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.; Kauffmann, H.M.; Ito, K.; Deeley, R.G.; Cole, S.P.; Schrenk, D. Identification of human multidrug resistance protein 1 (MRP1) mutations and characterization of a G671V substitution. J. Hum. Genet. 2001, 46, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoda, M.; Saito, Y.; Soyama, A.; Saeki, M.; Murayama, N.; Ishida, S.; Sai, K.; Nagano, M.; Suzuki, H.; Sugiyama, Y.; et al. Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: An association between single nucleotide polymorphisms in the 5′-untranslated region and exon 28. Drug Metab. Dispos. Biol. Fate Chem. 2002, 30, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Toh, S. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Fukuoka Igaku Zasshi (Hukuoka Acta Med.) 2000, 91, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Sha’ari, H.M.; Haerian, B.S.; Baum, L.; Saruwatari, J.; Tan, H.J.; Rafia, M.H.; Raymond, A.A.; Kwan, P.; Ishitsu, T.; Nakagawa, K.; et al. ABCC2 rs2273697 and rs3740066 polymorphisms and resistance to antiepileptic drugs in Asia Pacific epilepsy cohorts. Pharmacogenomics 2014, 15, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M.; von Stulpnagel, C.; Muhle, H.; Haenisch, S.; Remmler, C.; Majed, A.; Plischke, H.; Stephani, U.; Kluger, G.; Cascorbi, I. Impact of ABCC2 genotype on antiepileptic drug response in Caucasian patients with childhood epilepsy. Pharmacogenet. Genom. 2011, 21, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.F.; Wang, L.L.; Di, Y.M.; Xue, C.C.; Duan, W.; Li, C.G.; Li, Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 2008, 15, 1981–2039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sew, P.H.; Ambrose, H.; Ryan, S.; Chong, S.S.; Lee, E.J.; Lee, C.G. Nucleotide sequence analyses of the MRP1 gene in four populations suggest negative selection on its coding region. BMC Genom. 2006, 7, 111. [Google Scholar]
- Zhou, L.; Cao, Y.; Long, H.; Long, L.; Xu, L.; Liu, Z.; Zhang, Y.; Xiao, B. ABCB1, ABCC2, SCN1A, SCN2A, GABRA1 gene polymorphisms and drug resistant epilepsy in the Chinese Han population. Die Pharm. 2015, 70, 416–420. [Google Scholar]
- Rahsaz, M.; Azarpira, N.; Nikeghbalian, S.; Aghdaie, M.H.; Geramizadeh, B.; Moini, M.; Banihashemi, M.; Darai, M.; Malekpour, Z.; Malekhosseini, S.A. Association between tacrolimus concentration and genetic polymorphisms of CYP3A5 and ABCB1 during the early stage after liver transplant in an Iranian population. Exp. Clin. Transplant. 2012, 10, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Brambila-Tapia, A.J. MDR1 (ABCB1) polymorphisms: Functional effects and clinical implications. Rev. Investig. Clin. 2013, 65, 445–454. [Google Scholar]
- Ramirez-Bello, J.; Vargas-Alarcon, G.; Tovilla-Zarate, C.; Fragoso, J.M. Single nucleotide polymorphisms (SNPs): Functional implications of regulatory-SNP (rSNP) and structural RNA (srSNPs) in complex diseases. Gac. Med. Mex. 2013, 149, 220–228. [Google Scholar] [PubMed]
- Pratt, V.M.; Beyer, B.N.; Koller, D.L.; Skaar, T.C.; Flockhart, D.A.; Levy, K.D.; Vance, G.H. Report of new haplotype for ABCC2 gene: rs17222723 and rs8187718 in cis. J. Mol. Diagn. JMD 2015, 17, 201–205. [Google Scholar] [CrossRef] [PubMed]
- db SNP Database Homepage. Available online: http://www.ncbi.nlm.nih.gov/projects/SNP/ (accessed on 12 July 2018).
- Escalante-Santiago, D.; Feria-Romero, I.A.; Ribas-Aparicio, R.M.; Rayo-Mares, D.; Fagiolino, P.; Vazquez, M.; Escamilla-Nunez, C.; Grijalva-Otero, I.; Lopez-Garcia, M.A.; Orozco-Suarez, S. MDR-1 and MRP2 Gene Polymorphisms in Mexican Epileptic Pediatric Patients with Complex Partial Seizures. Front. Neurol. 2014, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Komatsu, H.; Higasa, K.; Takano, M.; Tsuchiya, K.; Hayashida, T.; Oka, S.; Gatanaga, H. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: A pharmacogenetic study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhou, B.T.; Yin, J.Y.; Xu, X.J.; Zhao, Y.C.; Lei, G.H.; Tang, Q.; Zhou, H.H.; Liu, Z.Q. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci. Ther. 2012, 18, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, S.M.; Desai, S.Y.; Marroni, M.; Cucullo, L.; Goodrich, K.; Bingaman, W.; Mayberg, M.R.; Bengez, L.; Janigro, D. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 2001, 42, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Giacomet, V.; Cattaneo, D.; Vigano, A.; Nannini, P.; Manfredini, V.; Ramponi, G.; Clementi, E.; Zuccotti, G.V. Tenofovir-induced renal tubular dysfunction in vertically HIV-infected patients associated with polymorphisms in ABCC2, ABCC4 and ABCC10 genes. Pediatr. Infect. Dis. J. 2013, 32, e403–e405. [Google Scholar] [CrossRef] [PubMed]
- Edavana, V.K.; Penney, R.B.; Yao-Borengasser, A.; Starlard-Davenport, A.; Dhakal, I.B.; Kadlubar, S. Effect of MRP2 and MRP3 Polymorphisms on Anastrozole Glucuronidation and MRP2 and MRP3 Gene Expression in Normal Liver Samples. Int. J. Cancer Res. Mol. Mech. 2015, 1, 1–18. [Google Scholar]
- Shringarpure, S.; Xing, E.P. Effects of sample selection bias on the accuracy of population structure and ancestry inference. G3 Genes Genomes Genet. 2014, 4, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Tonk, E.C.M.; Gurwitz, D.; Maitland-van der Zee, A.H.; Janssens, A. Assessment of pharmacogenetic tests: Presenting measures of clinical validity and potential population impact in association studies. Pharmacogenom. J. 2017, 17, 386–392. [Google Scholar] [CrossRef] [PubMed]

| Gene/Protein | Substrates | Inhibitors | Consequences of Mutation/Polymorphism |
|---|---|---|---|
| MRP1 (ABCC1) | Various glutathione, glucuronide, and sulfate conjugates, anthracyclines, vinca alkaloids, etoposide, teniposide, topotecan, SN-38, melphalan, methotrexate, non-organic heavy metal oxyanions, leukotriene C4 (LTC4), D4, E4. | Sulfinpyrazone, probenecid, MK571, LTC4, some Pgp inhibitors (e.g., cyclosporin A, verapamil, PSC 833) | Reduction in intracellular concentration of the drug reduced clearance. Association with resistance in cancer and infectious diseases |
| MRP2 (ABCC2) | bilirubin, cisplatin, pravastatin, sulforhodamine 101 acid chloride (Texas Red), GSH, GSH conjugates, glucuronide conjugates, Olmesartan, valsartan, vinblastine, vincristine, etoposide, methotrexate, cisplatin, rifampicin, sulfinpyrazone, ceftriaxone, camptothecins, mitoxantrone, saquinavir | MK571, furosemide, probenecid, protease inhibitor (ritonavir, saquinavir), nucleoside analog (lamivudine, abacavir, emtricitabine), nucleoside phosphonate (cidofovir, adefovir, tenofovir) | Reduced expulsion of bilirubin. Rapid decrease of substrate bioavailability and increased biliary excretion |
| Gene/Protein | Exon | Primer Direction | Longitude (bp) | Sequence (5′-3′) | Longitude Amplicon (bp) |
|---|---|---|---|---|---|
| MRP1 (ABCC1) [29] | 2 | Forward | 20 | GCAGAAGACACCACATACCT | 510 |
| Reverse | 20 | AGAAGAAGGAACTTAGGGTC | |||
| 10 | Forward | 18 | TCCTGGGCAGACAGATAG | 439 | |
| Reverse | 18 | TGAACCACAGCCGGAACT | |||
| 16 | Forward | 20 | GTTTAGTACAGTCTTGCCTT | 463 | |
| Reverse | 19 | CCAAAATCCTGCCTTCTAG | |||
| 17 | Forward | 21 | GTGGGCCAGCTGTTGTCTCGT | 441 | |
| Reverse | 20 | AGTGAGACCTGAGCCACACC | |||
| 23 | Forward | 20 | ATGCCTGGTTCATCATTATT | 514 | |
| Reverse | 20 | CTTTAGGTAACACTGGTATA | |||
| MRP2 (ABCC2) [23] | 10 | Forward | 21 | GGGTCCTAATTTCAATCCTTA | 310 |
| Reverse | 21 | TATTCTTCTGGGTGACTTTTT | |||
| 18 | Forward | 21 | GGAGTAGTGCTTAATATGAAT | 249 | |
| Reverse | 21 | CCCACCCCACCTTTATATCTT | |||
| 23 | Forward | 21 | TGCATGGTGCTGACAAAACTG | 218 | |
| Reverse | 21 | CACCACCTGACAGTTCTTGAG | |||
| 28 | Forward | 21 | TGCTACCCTTCTCCTGTTCTA | 269 | |
| Reverse | 21 | ATCCAGGCCTTCCTTCACTCC | |||
| 31 | Forward | 21 | AGGAGCTAACACATGGTTGCT | 272 | |
| Reverse | 21 | GGGTTAAGCCATCCGTGTCAA |
| Location | Polymorphism (n) | Allelic Count | Genotypic Count | HW 1 | 1000 G 2 | 1000 G Colombia 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| After Exon 2 | rs200647436 (45) | A | G | A/G | G/G | p-value | p-value | p | |
| 10(11.1%) | 80(88.9%) | 10 | 35 | 0.4 | 0 | 0.000003 | |||
| NG_028268.1:g.63440G>A (45) | A | G | A/G | G/G | p-value | p-value | p | ||
| 1(1.11%) | 89(98.8%) | 1 | 44 | 0.93 | - | - | |||
| NG_028268.1:g.63452G>A (45) | A | G | A/G | G/G | p-value | p-value | p | ||
| 85(94.4%) | 5 (5.5%) | 5 | 40 | 0.69 | - | - | |||
| rs200624910 (45) | C | G | C/G | G/G | p-value | CLINSEQ_SNP 4 p | |||
| 2(2.22%) | 88(97.7%) | 2 | 43 | 0.87 | 0.002 | ||||
| rs200647436: Count allelic 1000 Genomes: 4 (A)/5004 (G), Colombia allelic count: 188 (G). rs200624910: allelic CLINSEQ_SNP Count 3 (C)/1320 (G). NG_028268.1: g.63440G>A and NG_028268.1: g.63452G>A not been reported previously. | |||||||||
| After Exon 10 | NG_028268.1:g.103730T>G (41) | G | T | T/G | T/T | p-value | p-value | p-value | |
| 1(2.4%) | 81(98.8%) | 1 | 40 | 0.94 | - | - | |||
| NG_028268.1:g.103730T>G has not been reported previously. | |||||||||
| After Exon 16 | NG_028268.1:g.134922G>A (45) | A | G | A/G | G/G | p-value | p-value | p-value | |
| 2(2.22%) | 88(97.78%) | 2 | 43 | 0.87 | - | - | |||
| NG_028268.1:g.134927G>A (44) | A | G | A/G | G/G | p-value | p-value | p-value | ||
| 2(2.27%) | 86(97.73%) | 2 | 42 | 0.87 | - | - | |||
| NG_028268.1:g.134935G>A (44) | A | G | A/G | G/G | p-value | p-value | p-value | ||
| 1(1.14%) | 87(98.86%) | 1 | 43 | 0.93 | - | - | |||
| NG_028268.1:g.134940G>A (44) | A | G | A/G | G/G | p-value | p-value | p-value | ||
| 1(1.14%) | 87(98.86%) | 1 | 43 | 0.93 | - | - | |||
| NG_028268.1:g.134949T>A (44) | A | T | A/T | T/T | p-value | p-value | p-value | ||
| 1(1.14%) | 87(98.86%) | 1 | 43 | 0.93 | - | - | |||
| None of the polymorphisms has been reported previously. | |||||||||
| Exon 17 | NG_028268.1:g.138867G>A (46) | A | G | A/G | G/G | A/A | p-value | p-value | p-value |
| 5(5.43%) | 87(94.57%) | 1 | 43 | 2 | 0 | - | - | ||
| NG_028268.1:g.138902C>A (46) | A | C | A/C | C/C | A/A | p-value | p-value | p-value | |
| 1(1.09%) | 91(98.91%) | 1 | 45 | 0 | 0.94 | - | - | ||
| After Exon 17 | NG_028268.1:g.139009C>G (46) | C | G | C/G | G/G | C/C | p-value | p-value | p-value |
| 0(0%) | 46(100%) | 0 | 46 | 0 | - | - | - | ||
| None of the polymorphisms have been reported previously. | |||||||||
| Before Exon 23 | rs150214567 (43) | T | C | T/C | C/C | T/T | p-value | p-value | p-value |
| 0(0%) | 86(100%) | 0 | 43 | 0 | - | 0.85 | 0.5 | ||
| rs150214567: 1000 Genomes allelic count: 2 (T)/5006 (C), Colombia allelic count: 188 (C). | |||||||||
| Location | Polymorphism (n) | Allelic Count | Genotypic Count | HW 1 | 1000 G 2 | 1000 G Colombia 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| Exon 10 | rs2273697 (45) | A | G | A/G | G/G | A/A | p | p | p |
| 8(8.89%) | 82(91.11%) | 8 | 37 | 0 | 0.5 | 0.018 | 0.13 | ||
| rs2273697: 1000 Genomes allelic count: 934 (A)/4074 (G), Colombia allelic count: 29 (A)/159 (G) | |||||||||
| After Exon 18 | NG_011798.1:g.41261_A (28) | T | A | A/T | A/A | T/T | p | p | p |
| 14(25%) | 42(75%) | 14 | 14 | 0 | 0.07 | - | - | ||
| NG_011798.1:g.41261_A Has not been reported previously. In 13 individuals it was not possible to clearly determine the genotype. It is not possible to determine the full allele frequencies for forty individuals. | |||||||||
| Before Exon 23 | NG_011798.1:g.54253A>C (47) | A | C | A/C | A/A | C/C | p | p | p |
| I | I | I | I | I | I | - | - | ||
| NG_011798.1: g.54253A>C has not been reported previously. 18 subjects in genotype C/C was determined. In 29 subjects could not clearly establish the genotype. It is not possible to determine the full allele frequencies. | |||||||||
| Exon 28 | rs3740066 (49) | T | C | T/C | C/C | T/T | p | p | p |
| 84(85.71%) | 14(24.5%) | 14 | 0 | 35 | 0.24 | 0.01 | 0.01 | ||
| rs3740066: 1000 Genomes allelic count: 3565 (C)/1443 (T). Colombia allelic count: 121 (C)/67 (T). | |||||||||
| Exon 31 | rs142573385 (45) | T | C | T/C | C/C | T/T | p | p | p |
| 2(2.22%) | 88(97.78%) | 0 | 44 | 1 | 0 | 0 | 0.04 | ||
| rs142573385: 1000 Genomes allelic count: 5006 (C)/2 (T). Colombia allelic count: 188 (C). | |||||||||
| After Exon 31 | rs17216212 (43) | A (%) | G (%) | A/G | G/G | p | p | p | |
| - | 86(100%) | - | 43 | 0 | 0.06 | 0.12 | |||
| rs17216212: 1000 Genomes allelic count: 191 (A)/4817 (G). | |||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos-Cruz, R.H.; Martínez, L.R.; García, J.C.; Barreto, G.E.; Suárez, F. New ABCC2 rs3740066 and rs2273697 Polymorphisms Identified in a Healthy Colombian Cohort. Pharmaceutics 2018, 10, 93. https://doi.org/10.3390/pharmaceutics10030093
Bustos-Cruz RH, Martínez LR, García JC, Barreto GE, Suárez F. New ABCC2 rs3740066 and rs2273697 Polymorphisms Identified in a Healthy Colombian Cohort. Pharmaceutics. 2018; 10(3):93. https://doi.org/10.3390/pharmaceutics10030093
Chicago/Turabian StyleBustos-Cruz, Rosa Helena, Luis Rafael Martínez, Julio César García, George E. Barreto, and Fernando Suárez. 2018. "New ABCC2 rs3740066 and rs2273697 Polymorphisms Identified in a Healthy Colombian Cohort" Pharmaceutics 10, no. 3: 93. https://doi.org/10.3390/pharmaceutics10030093
APA StyleBustos-Cruz, R. H., Martínez, L. R., García, J. C., Barreto, G. E., & Suárez, F. (2018). New ABCC2 rs3740066 and rs2273697 Polymorphisms Identified in a Healthy Colombian Cohort. Pharmaceutics, 10(3), 93. https://doi.org/10.3390/pharmaceutics10030093