Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
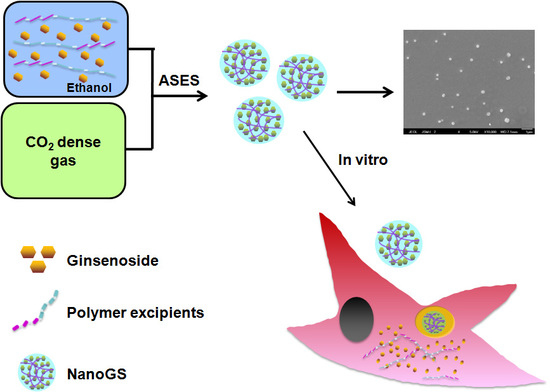
2.2. Synthesis of NanoGS
2.3. Morphology and Particle Size of NanoGS
2.4. Crystallinity of NanoGS
2.5. Dissolution Rate of NanoGS
2.6. In Vitro Anticancer Activity of NanoGS-Rh2
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of NanoGS
3.2. Crystallinity of NanoGS
3.3. Aqueous Stability and Dissolution Rate of NanoGS
3.4. In Vitro Anticancer Activity of NanoGS-Rh2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, K.W.; Wong, A.S.-T. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudakewich, M.; Ba, F.; Benishin, C.G. Neurotrophic and neuroprotective actions of ginsenosides Rb (1) and Rg (1). Planta Med. 2001, 67, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-C.; Zhu, Y.-G.; Zhu, L.-A.; Huang, C.; Chen, Y.; Chen, L.-M.; Fang, F.; Zhou, Y.-C.; Zhao, C.-H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-T.; Shao, Z.-H.; VandenHoek, T.L.; Chang, W.-T.; Li, J.; Mehendale, S.; Wang, C.-Z.; Hsu, C.-W.; Becker, L.B.; Yin, J.-J.; et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-S.; Liu, H.-C.; Yang, M.; Zuo, C.; Deng, Y.; Fan, J.-M. Ginsenoside Rb1, a panoxadiolsaponin against oxidative damage and renal interstitial fibrosis in rats with unilateral ureteral obstruction. Chin. J. Integr. Med. 2009, 15, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wu, L.; Li, C.-R.; Wang, X.-W.; Ma, Y.-J.; Zhong, Z.-Y.; Zhao, H.-B.; Cui, J.; Xun, S.-F.; Huang, X.-L.; et al. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J. Cell. Biochem. 2009, 108, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Cheung, L.W.T.; Pon, Y.L.; Wong, R.N.S.; Mak, N.K.; Fan, T.P.; Au, S.C.L.; Tombran-Tink, J.; Wong, A.S.T. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen β receptor. Br. J. Pharmacol. 2007, 152, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-G.; Sun, H.-X.; Ye, Y.-P. Ginsenoside Rd from panaxnotoginseng is cytotoxic towards hela cancer cells and induces apoptosis. Chem. Biodivers. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Popovich, D.G.; Hu, C. Characterizing the mechanism for ginsenoside-induced cytotoxicity in cultured leukemia (THP-1) cells. Can. J. Physiol. Pharmacol. 2007, 85, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Gong, X.-J.; Zheng, Y.-N. Isolation, synthesis and structures of cytotoxic ginsenoside derivatives. Molecules 2007, 12, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sun, S.; Xie, L.-H.; Wicks, S.M.; Xie, J.-T. Ginsenoside Re: Pharmacological effects on cardiovascular system. Cardiovasc. Ther. 2012, 30, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Zhou, X.-M.; Cao, Y.-L.; Hu, W.-X. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J. Asian Nat. Prod. Res. 2008, 10, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.-Z.; Wu, J.A.; Aung, H.H.; Rue, A.P.; Bell, G.I.; et al. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim. Biophys. Acta 2005, 1740, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Chen, J.; Sakwiwatkul, K.; Li, R.; Hu, S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 2010, 10, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effect of ginsenoside Re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J. Microbiol. Biotechnol. 2012, 22, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, P.; Gou, H.; Zhang, J.; Zhu, M.; Wang, Z.-H.; Tian, J.-W.; Jiang, Y.-T.; Fu, F.-H. Cardioprotective effects of 20(S)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.-H.; Xia, J.; Li, X.-P.; Li, K.-Q.; Xiong, W.; Li, J.; Chen, D.-L. 20(S)-ginsenoside Rh2 inhibits the proliferation and induces the apoptosis of KG-1a cells through the Wnt/β-catenin signaling pathway. Oncol. Rep. 2016, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Stegemann, S.; Leveiller, F.; Franchi, D.; de Jong, H.; Lindén, H. When poor solubility becomes an issue: From early stage to proof of concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kudo, S.; Takiyama, H. Development of simultaneous control of polymorphism and morphology in indomethacin crystallization. J. Cryst. Growth 2016, 435, 37–41. [Google Scholar] [CrossRef]
- Dwichandra Putra, O.; Umeda, D.; Fujita, E.; Haraguchi, T.; Uchida, T.; Yonemochi, E.; Uekusa, H. Solubility improvement of benexate through salt formation using artificial sweetener. Pharmaceutics 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Przybyłek, M. Selection of effective cocrystals former for dissolution rate improvement of active pharmaceutical ingredients based on lipoaffinity index. Eur. J. Pharm. Sci. 2017, 107, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, N.B.; Chowdary, K.P.R.; Murthy, K.V.R.; Satyanarayana, V.; Hayman, A.R.; Becket, G. Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary systems. J. Pharm. Biomed. Anal. 2004, 35, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lenz, E.; Jensen, K.T.; Blaabjerg, L.I.; Knop, K.; Grohganz, H.; Löbmann, K.; Rades, T.; Kleinebudde, P. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin–arginine. Eur. J. Pharm. Biopharm. 2015, 96, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Rieger, J. Organic nanoparticles in the aqueous phase—Theory, experiment, and use. Angew. Chem. Int. Ed. 2001, 40, 4330–4361. [Google Scholar] [CrossRef]
- Patel, Y.; Poddar, A.; Sawant, K. Formulation and characterization of Cefuroxime Axetilnanoemulsion for improved bioavailability. J. Pharm. Bioallied Sci. 2012, 4, S4–S5. [Google Scholar] [PubMed]
- Ali, H.S.M.; York, P.; Blagden, N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int. J. Pharm. 2009, 375, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadhead, J.; Edmond Rouan, S.K.; Rhodes, C.T. The spray drying of pharmaceuticals. Drug Dev. Ind. Pharm. 1992, 18, 1169–1206. [Google Scholar] [CrossRef]
- Li, X.-S.; Wang, J.-X.; Shen, Z.-G.; Zhang, P.-Y.; Chen, J.-F.; Yun, J. Preparation of uniform prednisolone microcrystals by a controlled microprecipitation method. Int. J. Pharm. 2007, 342, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, S.; Shen, H.; Qin, S.; Liu, J.; Zhang, Q.; Li, R.; Xu, Q. Diclofenac sodium-loaded solid lipid nanoparticles prepared by emulsion/solvent evaporation method. J. Nanoparticle Res. 2011, 13, 2375–2386. [Google Scholar] [CrossRef]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation-process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Charoenchaitrakool, M.; Dehghani, F.; Foster, N.R.; Chan, H.K. Micronization by rapid expansion of supercritical solutions to enhance the dissolution rates of poorly water-soluble pharmaceuticals. Ind. Eng. Chem. Res. 2000, 39, 4794–4802. [Google Scholar] [CrossRef]
- Domingo, C.; Berends, E.; van Rosmalen, G.M. Precipitation of ultrafine organic crystals from the rapid expansion of supercritical solutions over a capillary and a frit nozzle. J. Supercrit. Fluids 1997, 10, 39–55. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Tom, J.W.; Kwauk, X.; Yeo, S.D. Rapid expansion of supercritical solutions (RESS): Fundamentals and applications. Fluid Phase Equilibria 1993, 82, 311–321. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Di Trolio, A.; Pace, S. Supercritical antisolvent precipitation of nanoparticles of superconductor precursors. Ind. Eng. Chem. Res. 1998, 37, 952–958. [Google Scholar] [CrossRef]
- Bleich, J.; Müller, B.W. Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process. J. Microencapsul. 1996, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Meure, L.A.; Warwick, B.; Dehghani, F.; Regtop, H.L.; Foster, N.R. Increasing copper indomethacin solubility by coprecipitation with poly(vinylpyrrolidone) using the aerosol solvent extraction system. Ind. Eng. Chem. Res. 2004, 43, 1103–1112. [Google Scholar] [CrossRef]
- Tantishaiyakul, V.; Kaewnopparat, N.; Ingkatawornwong, S. Properties of solid dispersions of piroxicam in polyvinylpyrrolidone K-30. Int. J. Pharm. 1996, 143, 59–66. [Google Scholar] [CrossRef]
- Doherty, C.; York, P. Mechanisms of dissolution of frusemide/PVP solid dispersions. Int. J. Pharm. 1987, 34, 197–205. [Google Scholar] [CrossRef]
- Ma, G.; Song, C. PCL/poloxamer 188 blend microsphere for paclitaxel delivery: Influence of poloxamer 188 on morphology and drug release. J. Appl. Polym. Sci. 2007, 104, 1895–1899. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H.; Tóth, J.; Gyenis, J.; Feczkó, T. Poloxamers for surface modification of hydrophobic drug carriers and their effects on drug delivery. Polym. Rev. 2014, 54, 112–138. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, W.; Wang, J.; Foster, N.R.; Wen, N.; Zhang, J. Preparation of silybin/poly(vinylpyrrolidone) nanodrugs by using the aerosol solvent extraction system for improving drug solubility. Ind. Eng. Chem. Res. 2014, 53, 10519–10524. [Google Scholar] [CrossRef]
- Sarkari, M.; Brown, J.; Chen, X.; Swinnea, S.; Williams, R.O.; Johnston, K.P. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int. J. Pharm. 2002, 243, 17–31. [Google Scholar] [CrossRef]
- Lindfors, L.; Skantze, P.; Skantze, U.; Westergren, J.; Olsson, U. Amorphous drug nanosuspensions. 3. particle dissolution and crystal growth. Langmuir 2007, 23, 9866–9874. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Zhang, Z.-B.; Le, Y.; Zhao, H.; Chen, J.-F. A novel strategy to produce highly stable and transparent aqueous ‘nanosolutions’ of water-insoluble drug molecules. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Sethia, S.; Squillante, E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int. J. Pharm. 2004, 272, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jones, D.S.; Andrews, G.P. An investigation into the role of polymeric carriers on crystal growth within amorphous solid dispersion systems. Mol. Pharm. 2015, 12, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, C.; Zhang, J.; Wang, J.; Le, Y. Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability. Pharmaceutics 2018, 10, 95. https://doi.org/10.3390/pharmaceutics10030095
Tao C, Zhang J, Wang J, Le Y. Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability. Pharmaceutics. 2018; 10(3):95. https://doi.org/10.3390/pharmaceutics10030095
Chicago/Turabian StyleTao, Cheng, Jianjun Zhang, Jiexin Wang, and Yuan Le. 2018. "Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability" Pharmaceutics 10, no. 3: 95. https://doi.org/10.3390/pharmaceutics10030095
APA StyleTao, C., Zhang, J., Wang, J., & Le, Y. (2018). Ginsenoside Drug Nanocomposites Prepared by the Aerosol Solvent Extraction System for Enhancing Drug Solubility and Stability. Pharmaceutics, 10(3), 95. https://doi.org/10.3390/pharmaceutics10030095