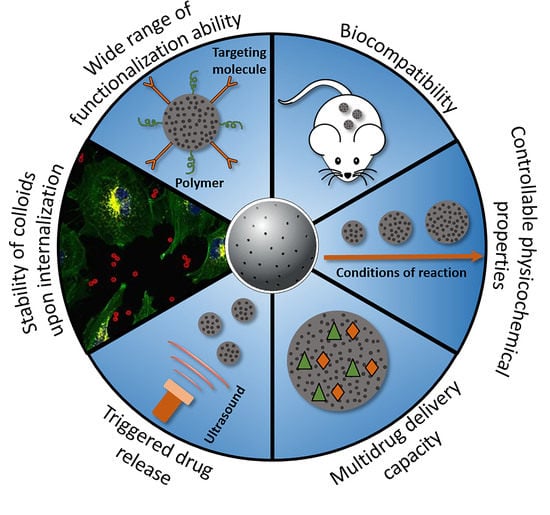
Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives
Abstract
:1. Introduction
Scope
2. Silica-Based Drug Carriers
2.1. Preparation Methods and Physical Chemistry of Silica-Based Carriers
2.2. Drug Loading Approaches
2.3. Release Efficiency
2.3.1. Internal Triggering
2.3.2. External Triggering
2.4. Delivery of Various Compounds In Vitro and In Vivo
3. Calcium Carbonate Drug Carriers
3.1. Synthesis and Loading of Drugs
3.2. Drug Loading
3.3. Drug Release
3.4. Delivery of Various Compounds In Vitro and In Vivo
4. Calcium Phosphate Carriers
4.1. Synthesis of Calcium Phosphate
4.2. Release Efficiency
4.3. In Vitro and In Vivo Delivery of Various Compounds with Calcium Phosphate Carriers
5. Concluding Remarks and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Meng, Q.; Chen, Y.; Du, Y.; Zhang, L.; Li, Y.; Zhang, L.; Shi, J. Large-Pore ultrasmall mesoporous organosilica nanoparticles: Micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv. Mater. 2015, 27, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Khashirova, S.Y.; Zhansitov, A.; Rumyantsev, E.V. Synthesis and application of silica hybrids grafted with new guanidine-containing polymers as highly effective adsorbents for bilirubin removal. Colloid Polym. Sci. 2015, 293, 1667–1674. [Google Scholar] [CrossRef]
- Timin, A.S.; Rumyantsev, E.V.; Solomonov, A.V.; Musabirov, I.I.; Sergeev, S.N.; Ivanov, S.P.; Berlier, G.; Balantseva, E. Preparation and characterization of organo-functionalized silicas for bilirubin removal. Colloids Surf. Physicochem. Eng. Asp. 2015, 464, 65–77. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Yan, B.-B.; Dong, L.; Lu, Y.; Yu, S.-H. Calcium carbonate-doxorubicin@silica-indocyanine green nanospheres with photo-triggered drug delivery enhance cell killing in drug-resistant breast cancer cells. Nano Res. 2018, 11, 3385–3395. [Google Scholar] [CrossRef]
- Timin, A.S.; Balantseva, E.V.; Khashirova, S.Y.; Rumyantsev, E.V.; Osadchaya, T.Y. Application of guanidine-containing polymers for preparation of pH responsive silica-based particles for drug delivery systems. Colloids Surf. Physicochem. Eng. Asp. 2015, 477, 26–34. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Shi, J.; Ma, T.; Zhang, Z.; Ma, H.; Cao, S. Hydroxyapatite/mesoporous silica coated gold nanorods with improved degradability as a multi-responsive drug delivery platform. Mater. Sci. Eng. C 2018, 83, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Man, M.W.C.; Durand, J.-O.; Khashab, N.M. Syntheses and applications of periodic mesoporous organosilica nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: Inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin. Drug Deliv. 2017, 14, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Khashirova, S.Y.; Rumyantsev, E.V.; Goncharenko, A.A. Magnetic silica hybrids modified with guanidine containing co-polymers for drug delivery applications. Mater. Sci. Eng. C 2016, 64, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.; Rumyantsev, E. Silver–Silica nanocomposite materials incorporated into textile fabrics: Chemical and biological study. BioNanoScience 2013, 3, 415–422. [Google Scholar] [CrossRef]
- Vassilakopoulou, A.; Georgakilas, V.; Vainos, N.; Koutselas, I. Successful entrapment of carbon dots within flexible free-standing transparent mesoporous organic-inorganic silica hybrid films for photonic applications. J. Phys. Chem. Solids 2017, 103, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Magnetic-responsive microparticles with customized porosity for drug delivery. RSC Adv. 2016, 6, 88157–88167. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Kantner, K.; Rejman, J.; Kraft, K.V.L.; Soliman, M.G.; Zyuzin, M.V.; Escudero, A.; Pino, P.D.; Parak, W.J. Laterally and temporally controlled intracellular staining by light-triggered release of encapsulated fluorescent markers. Chemistry 2018, 24, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Saprykina, N.N.; Sergeev, V.S.; Afanasyev, B.V.; Vilesov, A.D.; Sukhorukov, G.B. Triple-responsive inorganic-organic hybrid microcapsules as a biocompatible smart platform for the delivery of small molecules. J. Mater. Chem. B 2016, 4, 7270–7282. [Google Scholar] [CrossRef]
- Timin, A.S.; Gould, D.J.; Sukhorukov, G.B. Multi-layer microcapsules: Fresh insights and new applications. Expert Opin. Drug Deliv. 2017, 14, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Gao, H.; Voronin, D.V.; Gorin, D.A.; Sukhorukov, G.B. Inorganic/organic multilayer capsule composition for improved functionality and external triggering. Adv. Mater. Interfaces 2017, 4, 1600338. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Okilova, M.V.; Tcvetkov, N.Y.; Shakirova, A.I.; Afanasyev, B.V.; Gorin, D.A.; Sukhorukov, G.B. Intracellular breakable and ultrasound-responsive hybrid microsized containers for selective drug release into cancerous cells. Part. Part. Syst. Charact. 2017, 34, 1600417. [Google Scholar] [CrossRef]
- Croissant, J.; Cattoën, X.; Man, M.W.C.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable ethylene-bis(propyl)disulfide-based periodic mesoporous organosilica nanorods and nanospheres for efficient in-vitro drug delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Hühn, J.; Zyuzin, M.V.; Ashraf, S.; Valdeperez, D.; Masood, A.; Said, A.H.; Escudero, A.; Pelaz, B.; Gonzalez, E.; et al. Quantitative uptake of colloidal particles by cell cultures. Sci. Total Environ. 2016, 568, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M. Drug delivery from structured porous inorganic materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S.-Z. Disulfide-bridged organosilica frameworks: Designed, synthesis, redox-triggered biodegradation, and nanobiomedical applications. Adv. Funct. Mater. 2018, 28, 1707325. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J. Hollow-structured mesoporous materials: Chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Nie, H.; Wang, K.; Tan, W.; Wu, X.; Zhang, P. In Vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal. Chem. 2008, 80, 9597–9603. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Fukushima, Y. Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J. Mater. Chem. 2004, 14, 1579–1584. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizutani, M.; Nozaki, H.; Suzuki, N.; Yano, K. Formation mechanism for monodispersed mesoporous silica spheres and its application to the synthesis of core/shell particles. J. Phys. Chem. C 2007, 111, 1093–1100. [Google Scholar] [CrossRef]
- Möller, K.; Kobler, J.; Bein, T. Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 2007, 17, 605–612. [Google Scholar] [CrossRef]
- Yeh, Y.-Q.; Chen, B.-C.; Lin, H.-P.; Tang, C.-Y. Synthesis of hollow silica spheres with mesostructured shell using cationic-anionic-neutral block copolymer ternary surfactants. Langmuir 2006, 22, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Ruiz-Hernández, E.; Hennink, W.E.; Vallet-Regí, M. Magnetic mesoporous silica-based core/shell nanoparticles for biomedical applications. RSC Adv. 2013, 3, 9584–9593. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Kumagai, A.; Miyawaki, A.; Khashirova, S.Y.; Zhansitov, A.; Rumyantsev, E.V. Magnetic polymer-silica composites as bioluminescent sensors for bilirubin detection. Mater. Chem. Phys. 2016, 183, 422–429. [Google Scholar] [CrossRef]
- Ott, A.; Yu, X.; Hartmann, R.; Rejman, J.; Schütz, A.; Ochs, M.; Parak, W.J.; Carregal-Romero, S. Light-addressable and degradable silica capsules for delivery of molecular cargo to the cytosol of cells. Chem. Mater. 2015, 27, 1929–1942. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Rosenholm, J.M.; Gu, H. Synthesis and characterization of pore size-tunable magnetic mesoporous silica nanoparticles. J. Colloid Interface Sci. 2011, 361, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ying, J.Y. Generalized fluorocarbon-surfactant-mediated synthesis of nanoparticles with various mesoporous structures. Angew. Chem. 2005, 117, 292–296. [Google Scholar] [CrossRef]
- Du, B.; Cao, Z.; Li, Z.; Mei, A.; Zhang, X.; Nie, J.; Xu, J.; Fan, Z. One-pot preparation of hollow silica spheres by using thermosensitive poly (N-isopropylacrylamide) as a reversible template. Langmuir 2009, 25, 12367–12373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, P.; Chen, H.; Li, Y.; Bu, W.; Shu, Z.; Li, Y.; Zhang, J.; Zhang, L.; Pan, L.; et al. Colloidal HPMO nanoparticles: Silica-etching chemistry tailoring, topological transformation, and nano-biomedical applications. Adv. Mater. 2013, 25, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Q.; Wu, M.; Wang, S.; Xu, P.; Chen, H.; Li, Y.; Zhang, L.; Wang, L.; Shi, J. Hollow mesoporous organosilica nanoparticles: A generic intelligent framework-hybridization approach for biomedicine. J. Am. Chem. Soc. 2014, 136, 16326–16334. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Talented mesoporous silica nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Solomonov, A.V.; Musabirov, I.I.; Sergeev, S.N.; Ivanov, S.P.; Rumyantsev, E.V. Characterization and evaluation of silica particles coated by PVP and albumin for effective bilirubin removal. J. Sol-Gel Sci. Technol. 2015, 74, 187–198. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Musabirov, I.I.; Sergeev, S.N.; Ivanov, S.P.; Rumyantsev, E.V.; Goncharenko, A. Immobilization of bovine serum albumin onto porous poly (vinylpyrrolidone)-modified silicas. Ind. Eng. Chem. Res. 2014, 53, 13699–13710. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.-Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.-Y. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.; Rumyantsev, E.; Solomonov, A. Synthesis and application of amino-modified silicas containing albumin as hemoadsorbents for bilirubin adsorption. J. Non-Cryst. Solids 2014, 385, 81–88. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Q.; Li, C.; Bao, C.; Liu, Z.; Li, F.; Zhu, L. Anticancer drug release from a mesoporous silica-based nanophotocage regulated by either a one- or two-photon process. J. Am. Chem. Soc. 2010, 132, 10645–10647. [Google Scholar] [CrossRef] [PubMed]
- Mizoshita, N.; Tani, T.; Inagaki, S. Syntheses, properties and applications of periodic mesoporous organosilicas prepared from bridged organosilane precursors. Chem. Soc. Rev. 2011, 40, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Picard, S.; Aggad, D.; Klausen, M.; Mauriello, J.C.; Maynadier, M.; Mongin, O.; Clermont, G.; Genin, E.; Cattoën, X.; et al. Fluorescent periodic mesoporous organosilica nanoparticles dual-functionalized via click chemistry for two-photon photodynamic therapy in cells. J. Mater. Chem. B 2016, 4, 5567–5574. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.-F.; Feng, J. Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable ZnO quantum dots for intracellular drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.P.; Min, K.H.; Lee, H.J.; Jeong, S.Y.; Lee, S.C. pH-Tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs. Angew. Chem. Int. Ed. 2011, 50, 8853–8857. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Tziveleka, L.-A.; Varlas, S.; Iatrou, H. pH-Sensitive nanogates based on poly (l-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Maver, U.; Jemec, A.; Tišler, T.; Bele, M.; Dražić, G.; Benčina, M.; Pintar, A.; Planinšek, O.; Gaberšček, M. Hindered disulfide bonds to regulate release rate of model drug from mesoporous silica. ACS Appl. Mater. Interfaces 2013, 5, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Hu, J.-J.; Xu, Q.; Chen, S.; Jia, H.-Z.; Sun, Y.-X.; Zhuo, R.-X.; Zhang, X.-Z. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 39–44. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Tian, B.; Li, K.; Wang, L.; Liang, Y.; Han, J. Multifunctional mesoporous silica nanoparticles modified with tumor-shedable hyaluronic acid as carriers for doxorubicin. Colloids Surf. B Biointerfaces 2016, 144, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinšek, O.; Kros, A.; Gaberšček, M. Poly (propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Giménez, C.; de la Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Choi, E.S.; Cheon, J.Y.; Joo, S.H.; Ryu, J.-H. Noncovalent polymer-gatekeeper in mesoporous silica nanoparticles as a targeted drug delivery platform. Adv. Funct. Mater. 2015, 25, 957–965. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, K.; Liu, X.; Zhang, H. A new strategy to prepare glutathione responsive silica nanoparticles. RSC Adv. 2013, 3, 17700–17702. [Google Scholar] [CrossRef]
- Quignard, S.; Masse, S.; Laurent, G.; Coradin, T. Introduction of disulfide bridges within silica nanoparticles to control their intra-cellular degradation. Chem. Commun. 2013, 49, 3410–3412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardado-Alvarez, T.M.; Devi, L.S.; Russell, M.M.; Schwartz, B.J.; Zink, J.I. Activation of snap-top capped mesoporous silica nanocontainers using two near-infrared photons. J. Am. Chem. Soc. 2013, 135, 14000–14003. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Li, N.; Chen, D.; Qi, X.; Sha, W.; Jiao, Y.; Xu, Q.; Lu, J. Coumarin-containing photo-responsive nanocomposites for NIR light-triggered controlled drug release via a two-photon process. J. Mater. Chem. B 2013, 1, 5942–5949. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S. Red-light-responsive supramolecular valves for photocontrolled drug release from mesoporous nanoparticles. Langmuir 2016, 32, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Mondragón, L.; Ros-Lis, J.V.; Sancenón, F.; Marcos, M.D.; Martínez-Máñez, R.; Soto, J.; Pérez-Payá, E.; Amorós, P. Finely tuned temperature-controlled cargo release using paraffin-capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 11172–11175. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Zope, H.R.; Capell, M.B.; Kros, A. Coiled-coil peptide motifs as thermoresponsive valves for mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 9932–9934. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Leng, F.; Zheng, L.; Yang, J.; Wang, W.; Huang, C.Z. Autofluorescent and pH-responsive mesoporous silica for cancer-targeted and controlled drug release. Microporous Mesoporous Mater. 2014, 186, 187–193. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Li, P.-Z.; Nguyen, K.T.; Wang, X.-J.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, uniform, and redispersible mesoporous silica nanoparticles for cancer-targeted drug delivery in vivo. Adv. Funct. Mater. 2014, 24, 2450–2461. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Pan, L.; Shi, J. Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int. J. Nanomed. 2015, 11, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xu, Z.; Chen, Z.; Liu, X.; Hou, C.; Zhang, X.; Zhang, H. Fabrication of single-hole glutathione-responsive degradable hollow silica nanoparticles for drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 12600–12608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, H.; Yu, M.; Xu, C.; Liu, Y.; Tang, J.; Yang, Y.; Yu, C. Room temperature synthesis of dendritic mesoporous silica nanoparticles with small sizes and enhanced mRNA delivery performance. J. Mater. Chem. B 2018, 6, 4089–4095. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, D.; Hwang, B.H.; Munavalli, G.; Kauvar, A.; Lloyd, J.; Blomgren, R.; Faupel, L.; Meyer, T.; Mitragotri, S. Ultrasonic delivery of silica–gold nanoshells for photothermolysis of sebaceous glands in humans: Nanotechnology from the bench to clinic. J. Control. Release 2015, 206, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-κB pathways. Biomaterials 2010, 31, 8198–8209. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.O.; Grabinski, C.M.; Schrand, A.M.; Murdock, R.C.; Wang, W.; Gu, B.; Schlager, J.J.; Hussain, S.M. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009, 11, 15–24. [Google Scholar] [CrossRef]
- Park, E.-J.; Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009, 184, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cho, W.-S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009, 189, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Hu, S.-H.; Hsiao, C.-S.; Chen, Y.-Y.; Liu, D.-M.; Chen, S.-Y. Multifunctional magnetically removable nanogated lids of Fe3O4-capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J. Mater. Chem. 2011, 21, 2535–2546. [Google Scholar] [CrossRef]
- Horn, D.; Rieger, J. Organic nanoparticles in the aqueous phase—Theory, experiment, and use. Angew. Chem. Int. Ed. 2001, 40, 4330–4361. [Google Scholar] [CrossRef]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Spanos, N.; Koutsoukos, P.G. The transformation of vaterite to calcite: Effect of the conditions of the solutions in contact with the mineral phase. J. Cryst. Growth 1998, 191, 783–790. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Beck, R.; Andreassen, J.-P. The onset of spherulitic growth in crystallization of calcium carbonate. J. Cryst. Growth 2010, 312, 2226–2238. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Svenskaya, Y.I.; Fattah, H.; Zakharevich, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Parakhonskiy, B.V. Ultrasonically assisted fabrication of vaterite submicron-sized carriers. Adv. Powder Technol. 2016, 27, 618–624. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomão, R.; Costa, L.M.M.; Olyveira, G.M. Precipitated calcium carbonate nano-microparticles: Applications in drug delivery. Adv. Tissue Eng. Regen. Med. 2017, 3. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Tao, K.; Zhao, K.; Wang, J.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Influence of ovalbumin on CaCO3 precipitation during in vitro biomineralization. J. Phys. Chem. B 2010, 114, 5301–5308. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Chekroun, K.B.; Rodriguez-Navarro, C.; Gonzalez-Munoz, M.T.; Arias, J.M.; Cultrone, G.; Rodriguez-Gallego, M. Precipitation and growth morphology of calcium carbonate induced by myxococcus xanthus: Implications for recognition of bacterial carbonates. J. Sediment. Res. 2004, 74, 868–876. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Fujiwara, M.; Shiokawa, K.; Araki, M.; Ashitaka, N.; Morigaki, K.; Kubota, T.; Nakahara, Y. Encapsulation of proteins into CaCO3 by phase transition from vaterite to calcite. Cryst. Growth Des. 2010, 10, 4030–4037. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Bhanvase, B.A.; Sonawane, S.H.; Gogate, P.R.; Pandit, A.B. A novel approach for continuous synthesis of calcium carbonate using sequential operation of two sonochemical reactors. Ultrason. Sonochem. 2017, 35, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Saini, D.R.; Pandit, A.B. Continuous precipitation of calcium carbonate using sonochemical reactor. Ultrason. Sonochem. 2015, 24, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Biswas, P.; Achilefu, S. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 2016, 8, 12639–12647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Chen, S.-F.; Liu, L.; Yao, H.-B.; Qiu, Y.-H.; Gao, M.-R.; Yu, S.-H. Template-free polymorph discrimination and synthesis of calcium carbonate minerals. Chem. Commun. 2009, 5853–5855. [Google Scholar] [CrossRef] [PubMed]
- Stávek, J.; Hanslík, T.; Zapletal, V. Preparation of high-temperature superconductors by controlled double-jet precipitation. Mater. Lett. 1990, 9, 90–95. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Li, F.; Xie, B.; Qian, Y. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. J. Cryst. Growth 2002, 236, 357–362. [Google Scholar] [CrossRef]
- Huang, S.-C.; Naka, K.; Chujo, Y. Effect of molecular weights of poly (acrylic acid) on crystallization of calcium carbonate by the delayed addition method. Polym. J. 2008, 40, 154–162. [Google Scholar] [CrossRef]
- Qiu, N.; Yin, H.; Ji, B.; Klauke, N.; Glidle, A.; Zhang, Y.; Song, H.; Cai, L.; Ma, L.; Wang, G.; et al. Calcium carbonate microspheres as carriers for the anticancer drug camptothecin. Mater. Sci. Eng. C 2012, 32, 2634–2640. [Google Scholar] [CrossRef]
- Kitamura, M.; Konno, H.; Yasui, A.; Masuoka, H. Controlling factors and mechanism of reactive crystallization of calcium carbonate polymorphs from calcium hydroxide suspensions. J. Cryst. Growth 2002, 236, 323–332. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 2004, 14, 2073–2081. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; Teng, H.; Becker, U. Growth process and crystallographic properties of ammonia-induced vaterite. Am. Mineral. 2012, 97, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Du, W.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Facile synthesis of calcium carbonate with an absolutely pure crystal form using 1-butyl-3-methylimidazolium dodecyl sulfate as the modifier. Colloid Polym. Sci. 2013, 291, 2191–2202. [Google Scholar] [CrossRef]
- Ma, M.-G.; Su, R.-C. Biomineralization and biomimetic synthesis of biomineral and nanomaterials. In Advances in Biomimetics; Cavrak, M., Ed.; InTech: Shanghai, China, 2011; pp. 13–50. ISBN 978-953-307-191-6. [Google Scholar]
- Liu, Y.; Cui, Y.; Mao, H.; Guo, R. Calcium carbonate crystallization in the presence of casein. Cryst. Growth Des. 2012, 12, 4720–4726. [Google Scholar] [CrossRef]
- Manoli, F.; Kanakis, J.; Malkaj, P.; Dalas, E. The effect of aminoacids on the crystal growth of calcium carbonate. J. Cryst. Growth 2002, 236, 363–370. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.-S.; Zong, J.-Y.; Zhao, D.; Li, F.; Zhuo, R.-X.; Cheng, S.-X. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J. Phys. Chem. C 2010, 114, 18940–18945. [Google Scholar] [CrossRef]
- Feng, J.; Wu, G.; Qing, C. Biomimetic synthesis of hollow calcium carbonate with the existence of the agar matrix and bovine serum albumin. Mater. Sci. Eng. C 2016, 58, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 2018, 122, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gusliakova, O.; Atochina-Vasserman, E.N.; Sindeeva, O.; Sindeev, S.; Pinyaev, S.; Pyataev, N.; Revin, V.; Sukhorukov, G.B.; Gorin, D.; Gow, A.J. Use of submicron vaterite particles serves as an effective delivery vehicle to the respiratory portion of the lung. Front. Pharmacol. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Schmidt, S.; Fernandes, P.; Larionova, N.I.; Sukhorukov, G.B.; Duschl, C.; Möhwald, H.; von Klitzing, R. One-step formulation of protein microparticles with tailored properties: Hard templating at soft conditions. Adv. Funct. Mater. 2012, 22, 1914–1922. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Giorgi, M.L.D.; Nobile, C.; Rinaldi, R.; Leporatti, S. Control of colloidal CaCO3 suspension by using biodegradable polymers during fabrication. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 60–70. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Díez, P.; Goldsmith, M.; Carregal-Romero, S.; Teodosio, C.; Rejman, J.; Feliu, N.; Escudero, A.; Almendral, M.J.; Linne, U.; et al. Comprehensive and systematic analysis of the immunocompatibility of polyelectrolyte capsules. Bioconjug. Chem. 2017, 28, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Preisig, D.; Haid, D.; Varum, F.J.O.; Bravo, R.; Alles, R.; Huwyler, J.; Puchkov, M. Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur. J. Pharm. Biopharm. 2014, 87, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, Q.; Gao, C. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. Physicochem. Eng. Asp. 2010, 353, 132–139. [Google Scholar] [CrossRef]
- Xu, L. Sacrificial PSS-doped CaCO3 templates to prepare chitosan capsules and their deformation under bulk pressure. Polym. Bull. 2013, 70, 455–465. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Zyuzin, M.V.; Hussain, I.; Parak, W.J.; Carregal-Romero, S. Catalysis by multifunctional polyelectrolyte capsules. RSC Adv. 2016, 6, 81569–81577. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and Salt-mediated response of layer-by-layer assembled pss/pah microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Inclusion of proteins into polyelectrolyte microparticles by alternative adsorption of polyelectrolytes on protein aggregates. Biochemistry 2003, 68, 236–241. [Google Scholar] [PubMed]
- Kurapati, R.; Raichur, A.M. Composite cyclodextrin-calcium carbonate porous microparticles and modified multilayer capsules: Novel carriers for encapsulation of hydrophobic drugs. J. Mater. Chem. B 2013, 1, 3175–3184. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Foss, C.; Carletti, E.; Fedel, M.; Haase, A.; Motta, A.; Migliaresi, C.; Antolini, R. Tailored intracellular delivery via a crystal phase transition in 400 nm vaterite particles. Biomater. Sci. 2013, 1, 1273–1281. [Google Scholar] [CrossRef]
- Lauth, V.; Maas, M.; Rezwan, K. An evaluation of colloidal and crystalline properties of CaCO3 nanoparticles for biological applications. Mater. Sci. Eng. C 2017, 78, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Y.; Hu, Y.; Li, J.-P.; Dong, L.; Lin, L.-N.; Yu, S.-H. Synthesis of superparamagnetic CaCO3 mesocrystals for multistage delivery in cancer therapy. Small 2010, 6, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Yu, Q.; Hu, F.; Yuan, H. Taking advantage of the disadvantage: Employing the high aqueous instability of amorphous calcium carbonate to realize burst drug release within cancer cells. J. Mater. Chem. B 2017, 5, 2068–2073. [Google Scholar] [CrossRef]
- Wu, J.-L.; He, X.-Y.; Jiang, P.-Y.; Gong, M.-Q.; Zhuo, R.-X.; Cheng, S.-X. Biotinylated carboxymethyl chitosan/CaCO3 hybrid nanoparticles for targeted drug delivery to overcome tumor drug resistance. RSC Adv. 2016, 6, 69083–69093. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Tiourina, O.P.; Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Loading the multilayer dextran sulfate/protamine microsized capsules with peroxidase. Biomacromolecules 2003, 4, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2008, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Haase, A.; Antolini, R. Sub-micrometer vaterite containers: Synthesis, substance loading, and release. Angew. Chem. Int. Ed. 2012, 51, 1195–1197. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, W.; Li, H.; Shi, W.; Zhang, J.; Feng, J.; Yang, L. Facile green synthesis of calcium carbonate/folate porous hollow spheres for the targeted pH-responsive release of anticancer drugs. J. Mater. Chem. B 2016, 4, 5650–5653. [Google Scholar] [CrossRef]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zakaria, Z.A.B. A pH-sensitive, biobased calcium carbonate aragonite nanocrystal as a novel anticancer delivery system. BioMed Res. Int. 2013, 2013, 587451. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.-H.; Zhao, Y. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem. Int. Ed. 2015, 54, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.; Sergeev, R.; Lengert, E.; Zakharevich, A.; Parakhonskiy, B.; Gorin, D.; Sergeev, S.; Volodkin, D. Composite magnetite and protein containing CaCO3 crystals. External manipulation and vaterite calcite recrystallization-mediated release performance. ACS Appl. Mater. Interfaces 2015, 7, 21315–21325. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.N.; Trushina, D.B.; Marchenko, I.V.; Bukreeva, T.V. Calcium carbonate-based mucoadhesive microcontainers for intranasal delivery of drugs bypassing the blood-brain barrier. BioNanoScience 2016, 6, 261–268. [Google Scholar] [CrossRef]
- Ying, X.; Shan, C.; Jiang, K.; Chen, Z.; Du, Y. Intracellular pH-sensitive delivery CaCO3 nanoparticles templated by hydrophobic modified starch micelles. RSC Adv. 2014, 4, 10841–10844. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xie, H.; Su, B.-L.; Yao, B.; Yin, Y.; Li, S.; Chen, F.; Fu, Z. Calcium carbonate nanoplate assemblies with directed high-energy facets: Additive-free synthesis, high drug loading, and sustainable releasing. ACS Appl. Mater. Interfaces 2015, 7, 15686–15691. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Reddy, T.N.; Kumar, K.P.; Dhevendar, K.; Singh, S.; Amarnath, M.; Misra, S.; Rangari, V.K.; Rana, R.K. In situ strategy to encapsulate antibiotics in a bioinspired CaCO3 structure enabling pH-sensitive drug release apt for therapeutic and imaging applications. ACS Appl. Mater. Interfaces 2016, 8, 22056–22063. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Gobbo, O.L.; Ehrhardt, C.; Healy, A.M. Amorphous calcium carbonate-based-microparticles for peptide pulmonary delivery. ACS Appl. Mater. Interfaces 2016, 8, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, D.; Li, F.; Zhuo, R.-X.; Cheng, S.-X. Co-delivery of genes and drugs with nanostructured calcium carbonate for cancer therapy. RSC Adv. 2012, 2, 1820–18226. [Google Scholar] [CrossRef]
- Dong, Q.; Li, J.; Cui, L.; Jian, H.; Wang, A.; Bai, S. Using porous CaCO3/hyaluronic acid nanocages to accommodate hydrophobic photosensitizer in aqueous media for photodynamic therapy. Colloids Surf. Physicochem. Eng. Asp. 2017, 516, 190–198. [Google Scholar] [CrossRef]
- Wang, A.; Yang, Y.; Zhang, X.; Liu, X.; Cui, W.; Li, J. Gelatin-assisted synthesis of vaterite nanoparticles with higher surface area and porosity as anticancer drug containers in vitro. ChemPlusChem 2016, 81, 194–201. [Google Scholar] [CrossRef]
- Wu, J.-L.; Wang, C.-Q.; Zhuo, R.-X.; Cheng, S.-X. Multi-drug delivery system based on alginate/calcium carbonate hybrid nanoparticles for combination chemotherapy. Colloids Surf. B Biointerfaces 2014, 123, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, G.; Yu, W.; Li, L.; Tong, Z.; Kong, X.; Yao, J. Oral delivery of insulin using CaCO3-based composite nanocarriers with hyaluronic acid coatings. Mater. Lett. 2017, 188, 263–266. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, P.; Sun, Y.; Gong, T.; Zhu, M.; Sun, X.; Zhang, Z.; Duan, Y. Preparation and properties of biocompatible PS-PEG/calcium phosphate nanospheres. Nanotoxicology 2015, 9, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Live-cell imaging to compare the transfection and gene silencing efficiency of calcium phosphate nanoparticles and a liposomal transfection agent. Gene Ther. 2017, 24, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Li, L.; Howard, C.B.; Mahler, S.M.; Huang, L.; Xu, Z.P. Preparation of optimized lipid-coated calcium phosphate nanoparticles for enhanced in vitro gene delivery to breast cancer cells. J. Mater. Chem. B 2015, 3, 6805–6812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoğlu, E.İ.; Tabaković, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hunziker, E.; Randall, N.; de Groot, K.; Layrolle, P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials 2003, 24, 65–70. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.-J.; Zhao, X.-Y.; Lu, B.-Q.; Tang, Q.-L.; Zhao, J.; Chen, F. Highly stable amorphous calcium phosphate porous nanospheres: Microwave-assisted rapid synthesis using ATP as phosphorus source and stabilizer, and their application in anticancer drug delivery. Chemistry 2013, 19, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-Dependent, Solution-mediated, solid-solid conversion. J. Phys. Chem. 1973, 77, 2313–2317. [Google Scholar] [CrossRef]
- Kandori, K.; Noguchi, Y.; Fukusumi, M.; Morisada, Y. Preparation of spherical and balloonlike calcium phosphate particles from forced hydrolysis of Ca(OH)2-triphosphate solution and their adsorption selectivity of water. J. Phys. Chem. C 2010, 114, 6440–6445. [Google Scholar] [CrossRef]
- Ding, G.-J.; Zhu, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous hollow microspheres of amorphous calcium phosphate: Soybean lecithin templated microwave-assisted hydrothermal synthesis and application in drug delivery. J. Mater. Chem. B 2015, 3, 1823–1830. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Inuoe, M.; Yusa, S.; Liao, S.-H.; Wu, K.C.-W.; Nakashima, K.; Yamauchi, Y. A block copolymer micelle template for synthesis of hollow calcium phosphate nanospheres with excellent biocompatibility. Chem. Commun. 2012, 48, 6532–6534. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.-H.; Zein, S.H.S.; Ahmad, A.L.; McPhail, D.S. Comparison of DOPA and DPPA liposome templates for the synthesis of calcium phosphate nanoshells. Ceram. Int. 2012, 38, 561–570. [Google Scholar] [CrossRef]
- Schmidt, H.T.; Gray, B.L.; Wingert, P.A.; Ostafin, A.E. Assembly of aqueous-cored calcium phosphate nanoparticles for drug delivery. Chem. Mater. 2004, 16, 4942–4947. [Google Scholar] [CrossRef]
- Schmidt, H.T.; Ostafin, A.E. Liposome directed growth of calcium phosphate nanoshells. Adv. Mater. 2002, 14, 532–535. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, K.; Li, Z.; Huang, S. Synthesis of hollow hybrid hydroxyapatite microspheres based on chitosan-poly (acrylic acid) microparticles. Biomed. Mater. 2009, 4, 031002. [Google Scholar] [CrossRef] [PubMed]
- Kandori, K.; Tada, K.; Fukusumi, M.; Morisada, Y. Preparation and characterization of spherical and balloon-like calcium phosphate particles. Bull. Chem. Soc. Jpn. 2008, 81, 1567–1574. [Google Scholar] [CrossRef]
- Sokolova, V.V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Pan, H.; Xu, X.; Hu, Q.; Li, L.; Tang, R. Ultrasonic controlled morphology transformation of hollow calcium phosphate nanospheres: A smart and biocompatible drug release system. Chem. Mater. 2007, 19, 3081–3083. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Huang, L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Control. Release 2012, 158, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauro, V.D.; Iafisco, M.; Salvarani, N.; Vacchiano, M.; Carullo, P.; Ramírez-Rodríguez, G.B.; Patrício, T.; Tampieri, A.; Miragoli, M.; Catalucci, D. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of microRNAs. Nanomedicine 2016, 11, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Haynes, M.T.; Wang, Y.; Liu, F.; Huang, L. A highly efficient synthetic vector: Nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano 2013, 7, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Bassett, D.C.; McKee, M.D.; Nelea, V.; Gbureck, U.; Barralet, J.E. The importance of particle size and DNA condensation salt for calcium phosphate nanoparticle transfection. Biomaterials 2008, 29, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Jain, S.; Tsai, P.-C.; Margolis, H.C.; Amiji, M. Nano-sized calcium phosphate particles for periodontal gene therapy. J. Periodontol. 2013, 84, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Deng, W.; Wei, Y.; Su, W.; Yang, Y.; Wei, Y.; Yu, J.; Xu, X. Encapsulation of plasmid DNA in calcium phosphate nanoparticles: Stem cell uptake and gene transfer efficiency. Int. J. Nanomed. 2011, 2011, 3335–3349. [Google Scholar] [CrossRef]
- Qiu, C.; Wei, W.; Sun, J.; Zhang, H.-T.; Ding, J.-S.; Wang, J.-C.; Zhang, Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale 2016, 8, 13033–13044. [Google Scholar] [CrossRef] [PubMed]
- Frede, A.; Neuhaus, B.; Klopfleisch, R.; Walker, C.; Buer, J.; Müller, W.; Epple, M.; Westendorf, A.M. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J. Control. Release 2016, 222, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kennell, C.; Lee, J.-Y.; Leung, Y.-K.; Tarapore, P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tanaka, Y.; Czernuszka, J.T. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials 2007, 28, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Kuhn, L. Chemotherapy drug delivery from calcium phosphate nanoparticles. Int. J. Nanomed. 2007, 2, 667–674. [Google Scholar]
- Haedicke, K.; Kozlova, D.; Gräfe, S.; Teichgräber, U.; Epple, M.; Hilger, I. Multifunctional calcium phosphate nanoparticles for combining near-infrared fluorescence imaging and photodynamic therapy. Acta Biomater. 2015, 14, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tabaković, A.; Kester, M.; Adair, J.H. Calcium phosphate-based composite nanoparticles in bioimaging and therapeutic delivery applications: Nanoparticles in bioimaging and therapeutic delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Altınoğlu, E.İ.; Adair, J.H. Near Infrared imaging with nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Bhakta, G.; Mitra, S.; Maitra, A. pDNA loaded calcium phosphate nanoparticles: Highly efficient non-viral vector for gene delivery. Int. J. Pharm. 2005, 288, 157–168. [Google Scholar] [CrossRef] [PubMed]









| Synthesis Method | Particle Size | Active Drug | In Vitro/In Vivo Results | Ref. |
|---|---|---|---|---|
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. Calcination was performed at 550 °C for 6 h. | 100 nm | Doxorubicin | The folate-receptor-mediated targeted delivery was proved by qualitative confocal laser scanning microscopy (CLSM) measurement. Cellular uptake of the doxorubicin-loaded nanoparticles in folate receptor-overexpressing HeLa cells was detected to be much higher than that in non-folate receptor-overexpressing adenocarcinomic human alveolar basal epithelial cells (A549) cells. | [74] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. Collect precipitate, wash with water and methanol, dry with vacuum. Resuspend particles in methanol, HCl and stir at 60 °C for 48 h. | 200 nm | Doxorubicin | The human liver cancer cell line (HepG2) cells viability with drug-loaded ZnO@MSN decreased below 50% at a concentration of 50 µg/mL, which is two times lower than that viability of cells incubated with free doxorubicin. | [55] |
| Template method Disperse polystyrene nanoparticles functionalized with sulfonic groups in ethanol and ammonia. Add TEOS and stir under 50 °C for 3 h. Soak polystyrene nanoparticles with tetrahydrofuran. | 200 nm | Doxorubicin | Synthesized GSH-responsive hollow silica nanoparticles showed near 3-fold higher doxorubicin release at pH 5.0 than at pH 7.4 without the presence of GSH. Qualitative CLSM observations confirmed that doxorubicin-loaded nanocarriers were internalized and the drug was released to reach cell nuclei within 24 h. | [77] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. To remove CTAB, reflux in HCl and methanol for 16 h. | 150 nm | Doxorubicin | Synthesized GSH responsive silica nanoparticles capped with peptide incubated separately with avβ3-integrin-positive tumor cell line (U87 MG) and avβ3-integrin-negative cell line (COS 7) at a doxorubicin concentration of 2 µg mL−1 possessed 1.7-fold higher U87 MG cellular uptake than that of silica nanoparticles without peptide. There was very little difference between the carriers in COS 7. | [60] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. To remove CTAB, add ethanol and HCl at 60 °C for 24 h. | 40 nm | Doxorubicin | Hyaluronic acid modified silica-based nanocarriers showed redox-responsive drug release and high drug loading (14%). The developed carriers were internalized in 2-fold higher amounts in HeLa cells than in LO2 cells. | [61] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. Calcination was performed at 550 °C for 5 h. | 100 nm | Doxorubicin and dye | GSH-responsive PEG-capped silica-based particles were used to deliver safranin O and doxorubicin in a controlled manner in vitro, achieving 90% of the maximum release of the entrapped drug in less than 1 h. The results showed that the PEG-capped systems were closed at low GSH concentrations. The cargo was released/delivered when the concentration of GSH increased. | [63] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. Calcination was performed at 550 °C for 5 h. | 150 nm | Cisplatin and doxorubicin | Polymer-gatekeeper mesoporous silica nanoparticles were synthesized by noncovalent capping of the pores of drug-loaded nanocontainers with disulfide cross-linkable polymers. By varying crosslinking density from 19% to 83%, the drug release was decreased almost in 2-fold. | [64] |
| Template method Add TEOS to the polystyrene latex nanoparticles. Calcination was performed at 600 °C to remove polystyrene nanoparticles. | 150 nm | Doxorubicin | The in vitro experiments indicated that the silica-based nanocarriers modified by near infrared (NIR)-light responsive polymers had a considerable drug loading efficiency of more than 70%. A significant number of drug molecules (>50%) could be released from the nanocarriers upon NIR-light irradiation. | [69] |
| Mix CTAB with NaOH solution. Add TEOS and APTES at 80 °C to the surfactant solution. Wash with ethanol. | 100 nm | Doxorubicin | A novel multifunctional envelope-type mesoporous silica nanoparticle system was used for drug delivery and magnetic resonance imaging (MRI) in vivo. The doxorubicin release was markedly increased under acidic conditions; more than 60% and 90% of the drug were released at pH 5.0 and pH 2.0, respectively. | [87] |
| Sol-gel method Mix CTAB with NaOH solution. Add TEOS at 80 °C to the surfactant solution. To remove CTAB, reflux in HCl and methanol for 24 h. | 100 nm | FITC dye, (S)–(+)– camptothecin | The cumulative drug release from the synthesized MSN@Fe3O4 nanocarriers was increased from 0.2% to about 21.9% over a 5 min magnetic stimulus. Obtained carriers showed T2-type MR contrast enhancement for cell or molecular imaging. | [88] |
| Synthesis Method | Particle Size | Active Drug | In Vitro/In Vivo Results | Ref. |
|---|---|---|---|---|
| CO2 diffusion through CaCl2 in EtOH | 100 nm | No drug | Resulted carriers modulated local pH and repeated daily administration of nano-CaCO3 inhibit tumor growth up to 2-fold in comparison with control. Efficient alkalization of the acidic pH of tumors depended on the particle size. | [106] |
| CaCl2 + NaHCO3 1:5 DI H2O:polyethylene glycol Mix for 5 min | 20 nm | |||
| CaCl2 + NaHCO3 1:5 DI H2O:ethylene glycol Mix for 30 min | 300 nm | |||
| NH4HCO3 vapor diffusion through CaCl2 in EtOH oleic acid stabilizer and polyethylene glycol corona | 600 nm | Doxorubicin | ACC particles with an oleic acid shell and a polyethylene glycol (PEG) corona showed retarded drug release profile with merely 14% of the drug being released after 24 h, decreased pH value up to 5.5 did not drastically increase the drug release, revealing that the drug locking effect was well realized. | [136] |
| CaCl2 + NaHCO3 water:gelatin:ethylene glycol Mix for 2 h | 250 nm | photosensitizer Hypocrellin B | The cellular internalization of hybrid nanoparticles modified by cross-linked hyaluronic acid by MCF-7 cells overexpressing cell-surface glycoprotein (CD44 receptor) has been enhanced from 2.2% to 85%, endowing the nanoparticles with targeting functionality. | [153] |
| CaCl2 + NaHCO3 starch solution Mix for 10 min | 800 nm | Doxorubicin, Au–DNA | Qualitative CLSM observations showed efficient intracellular delivery of doxorubicin by the CaCO3 carriers and especially into the nuclei of A549 and HeLa cells. | [135] |
| Polypeptide mediated mineralization from CaCl2 + (NH4)2CO3 | 500–1000 nm | Tetracycline | The IC50 (half maximal inhibitory concentration) values for various cell lines indicated relatively greater cell inhibition (from 1.8 to 8 fold) in the case of all the three cancer cell lines (A549, epithelial human breast cancer cell line (MDA-MB-231) and HeLa) in comparison to the normal cells. | [150] |
| CaCl2 + NaHCO3 ethylene glycol (EG), gelatin Mix for 2 h | 230 nm | Doxorubicin | Vaterite nanoparticles embedded with folic acid containing doxorubicin exhibited 2-fold higher cytotoxicity to MCF-7 cells compared with that of drug-loaded vaterite particle and free drug at concentrations of 0.02 and 0.04 mg/mL. | [154] |
| Spray Drying (NH4)2CO3 + Ca(OH)2 Hyaluronate polysaccharide | 4600 nm | Salmon calcitonin, alpha-1-antitrypsin protein | The bioavailability of salmon calcitonin after aerosol delivery as peptide-loaded composite microparticles to rats was 4-times higher than that of salmon calcitonin solution. | [151] |
| Starch solution and starch-octanoic acid were set as templates to prepare CaCO3 nanoparticles from CaCl2 + NaHCO3 Mix for 30 min | 400–500 nm | Doxorubicin | The IC50 of drug-loaded nanoparticles synthesized using 0.0625% starch-octanoic was almost 24-fold higher than that of DOX·HCl indicating higher A549 cellular uptake and faster drug release at the acidic pH. | [148] |
| CaCl2 + NaHCO3 1–2% hyaluronic acid | 150–350 nm | Insulin | An effective hypoglycemic effect was obtained in vivo compared with subcutaneous injection of insulin. After oral administration of insulin-loaded CaCO3 the blood glucose level is decreased 2-times slower for 3 h than that of with using insulin solution. | [156] |
| CaCl2 + NaHCO3 sodium alginate | 200 nm | Doxorubicin, paclitaxel | Qualitative CLSM observations showed that the dual drug loaded nanoparticles exhibited significantly enhanced cell uptake and nuclear localization as compared with the single drug loaded nanoparticles. | [155] |
| CaCl2 + Na2CO3 chitosan | >200 nm | Doxorubicin, P-glycoprotein inhibitor (tariquidar) | Enhanced multidrug-resistant breast cancer (MCF-7) cells uptake and nuclear localization were observed qualitatively for the drug-loaded nanoparticles by CLSM. Drugs co-delivery systems demonstrated near 2-fold higher cell inhibition rates compared with doxorubicin delivery systems. | [137] |
| Synthesis Method | Particle Size | Active Drug | In Vitro/In Vivo Results | Ref. |
|---|---|---|---|---|
| Water-in-oil micro-emulsion method CaCl2 (in Cyclohexane/Igepal) + Na2HPO4 (pH = 9.0) in oil Mix for 20 min | 40 nm | siRNA | A 40-fold improved silence activity compared to the previous analogous formulations. Reported nanoparticle vehicles effectively delivered siRNA to a solid tumor in a xenograft model in vitro and in vivo. | [182] |
| CaCl2 + Na3Cit + Na2HPO4 at pH 8.5 Mix for 5, 10, 20, 60 min at 37 °C | 20–50 nm | miRNA | Calcium phosphate nanoparticles efficiently internalized into cardiomyocytes. Dose-response graphs are given. The nanoparticles did not show promoting toxicity or interfering with any functional properties. Nanoparticles successfully encapsulated synthetic miRNAs, which were efficiently delivered into cardiac cells in vitro and in vivo. | [183] |
| CaCl2 (in Cyclohexane/Igepal) + Na2HPO4 (pH = 9.0) in CHCl3 | 52–56 nm | DNA | Resulted calcium phosphate carriers showed multifunctional features. PEGylation of these carriers enabled delivery to hepatocytes in vivo. Co-delivery of cationic peptides CR8C supported extensive nuclear translocation of DNA in post-mitotic cells. Monocyclic CR8C significantly enhanced in vivo gene expression over 10-fold more than linear CR8C. Carriers had improved stability and protecte DNA from degradation, though 100-fold lower in gene expression was detected, the developed system presents a greatly decreased invasiveness in its application. | [184] |
| CaCl2 in bis (2-ethylhexyl) sulphosuccinate (in hexane) + Na2HPO4 in bis (2-ethylhexyl) sulphosuccinate Mix overnight at 4 °C | 100–120 nm | DNA | Resulted carriers showed the transfection efficiency of 3% higher than that from the commercial transfecting reagent Polyfect. | [197] |
| Ca(NO3)2 + (NH4)3PO4 precipitated using a Harvard 22 syringe pump | 20–150 nm | DNA | Effective transfection with calcium phosphate particles in different cell types was demonstrated. | [186] |
| CaCl2 (in Cyclohexane/Igepal) + Na2HPO4 (in Tris-HCl/Cyclohexane/Igepal) Mix for 10 min at 4 °C | 20–50 nm | DNA | Resulted DNA carriers showed 5.7% lower cytotoxicity than commercial reagent Lipofectamine 2000. It has been demonstrated that calcium phosphate nanoparticles can be developed into an effective alternative as a non-viral gene delivery system. | [188] |
| CaCl2 + H24Na3O16P (in NaCl/KCl/dextrose/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) Syringe pump | 30–50 nm | DNA and growth factors | Synthesized calcium phosphate particles showed higher levels of biocompatibility (between 84% and 95% of mouse embryo (NIH-3T3) fibroblasts viability) and transfection efficiency into fibroblasts in vitro. Enzyme-linked Immunosorbent Assay (ELISA) test showed 3-fold higher platelet-derived (PDGF-B) growth factor expression in NIH-3T3 fibroblast cell line upon administration of nanocarriers than that of other applied substances. | [187] |
| CaCl2 + Alendronate-hyaluronic acid conjugate (in HEPES) | 170 nm | siRNA | Resulted calcium phosphate carriers showed effectively delivered siRNA in the A549 cells and contributed to the gene silencing (with the efficiency of about 40%) in vivo and in vitro. | [189] |
| C6H12CaO6 + (NH4)2HPO4 | 150 nm | siRNA | Particles exhibited rapid cellular uptake, almost no toxicity, and reduced gene expression of approximately 50% compared to the controls. A specific knockdown of target genes at the site of inflammation was achieved. | [190] |
| CaCl2 (in Igepal/Cyclohexane) + Na2HPO4 (in Igepal/Cyclohexane/DOPA in chloroform) | 100 nm | Paclitaxel and miRNA-221/222 inhibitors | Carriers loaded with multiple drugs simultaneously delivered paclitaxel and miRNA-221/222 inhibitors to their intracellular targets, leading to inhibit proliferative mechanisms of mRNA-221/222 with further enhancing the therapeutic efficacy of paclitaxel. It was demonstrated that the co-delivery nanocarrier system had 80% efficiency of tumor cell suppression when compared to free paclitaxel or delivering nanocarrier with a single drug (i.e., miRi only or paclitaxel only). | [191] |
| Ca(NO3)2 + K2HPO4 Stir for 1 h | 129 nm | Cisplatin | Synthesized calcium phosphate nanoparticles were non-toxic. Drug-loaded nanoparticles showed comparable cytotoxicity to free drug in an in vitro cell proliferation assay using the cisplatin-resistant human ovarian carcinoma (A2780cis) cell line. Negatively charged drug nanoconjugates are unable to overcome drug resistance and had the 4-fold increase in IC50 value as compared to the free drug. | [193] |
| C6H10CaO6 + (NH4)2HPO4 Stir for 20 min | 200 nm | Temoporfin, cyclic Arginine-Glycine-Aspartic acid-Phenylalanine-Lysine (RGDfK) peptide, fluorescent dye | Efficient drug delivery resulted in 2 times decrease in tumor vascularization in 1 week of treatment in vivo. Synthesized carriers combined diagnostic imaging, tumor targeting and drug delivery properties. | [194] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. https://doi.org/10.3390/pharmaceutics10040167
Trofimov AD, Ivanova AA, Zyuzin MV, Timin AS. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics. 2018; 10(4):167. https://doi.org/10.3390/pharmaceutics10040167
Chicago/Turabian StyleTrofimov, Alexey D., Anna A. Ivanova, Mikhail V. Zyuzin, and Alexander S. Timin. 2018. "Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives" Pharmaceutics 10, no. 4: 167. https://doi.org/10.3390/pharmaceutics10040167
APA StyleTrofimov, A. D., Ivanova, A. A., Zyuzin, M. V., & Timin, A. S. (2018). Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics, 10(4), 167. https://doi.org/10.3390/pharmaceutics10040167