Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO3 Nanofibers with a Mesoporous Structure as Drug Delivery Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
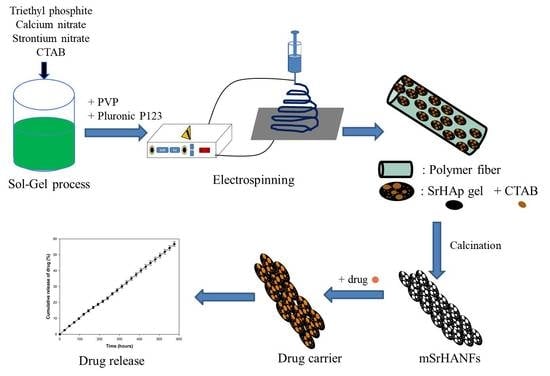
2.2. Synthesis and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CO3 Nanofibers Containing with a Mesoporous Structure (mSrHANFs)
2.3. Characterization of the mSrHANFs
2.4. In Vitro Study of the Degradability of mSrHANFs
2.5. In Vitro Study of Drug Loading and Release
2.6. Antibiotic Activity against Staphylococcus Aureus and Pseudomonas Aeruginosa
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Chang, Y.H.; Yu, J.L.; Hsu, H.W.; Rau, L.R.; Hsu, F.Y. Preparation of nanofibrous structure of mesoporous bioactive glass microbeads for biomedical applications. Materials 2016, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Lu, M.R.; Weng, R.C.; Lin, H.M. Hierarchically biomimetic scaffold of a collagen-mesoporous bioactive glass nanofiber composite for bone tissue engineering. Biomed. Mater. 2015, 10, 025007. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, T.W.; Qi, C.; Ding, Z.; Zhao, H.; Chen, F.; Chen, D.; Zhu, Y.J.; Shi, Z.; He, Y. Strontium-doped amorphous calcium phosphate porous microspheres synthesized through a microwave-hydrothermal method using fructose 1,6-bisphosphate as an organic phosphorus source: Application in drug delivery and enhanced bone regeneration. ACS Appl. Mater. Interface 2017, 9, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A. Biomimetic Mg- and Mg, CO3-substituted hydroxyapatites: Synthesis characterization and in vitro behavior. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Preparation and characterization of magnesium/carbonate co-substituted hydroxyapatites. J. Mater. Sci. Mater. Med. 2002, 13, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Acchar, W.; Ramalho, E.G. Effect of MnO2 addition on sintering behavior of tricalcium phosphate: Preliminary results. Mater. Sci. Eng. C. 2008, 28, 248–252. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.; Darvell, B.W.; Lu, W.W.; Chang, J.; Deng, L.; Wang, D.; et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, G.; Luk, K.D.; Cheung, K.M.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhang, B.; Zhu, X.; Ao, P.; Guo, H.; Yi, W.; Zhou, G.Q. Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. Biomed. Res. Int. 2014, 2014, 814057. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin. Oral Implants Res. 2016, 27, e206–e214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Guo, X.; Cui, W.; Yang, S.; Wang, J.; Qu, Y.; Shao, Z.; Xu, S. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed. Mater. 2017, 13, 015018. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; He, X.; Wu, Z. Mesoporous hydroxyapatite: Preparation, drug adsorption, and release properties. Mater. Chem. Phys. 2014, 148, 153–158. [Google Scholar] [CrossRef]
- Shyong, Y.J.; Wang, M.H.; Kuo, L.W.; Su, C.F.; Kuo, W.T.; Chang, K.C.; Lin, F.H. Mesoporous hydroxyapatite as a carrier of olanzapine for long-acting antidepression treatment in rats with induced depression. J. Control. Release 2017, 255, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Yin, M.; Yang, X.; Yuan, Q.; Ren, J.; Qu, X. Aptamer-capped multifunctional mesoporous strontium hydroxyapatite nanovehicle for cancer-cell-responsive drug delivery and imaging. Biomacromolecules 2012, 13, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, D.P.; Ye, S.; Zhao, X. Strontium-substituted, luminescent and mesoporous hydroxyapatite microspheres for sustained drug release. J. Mater. Sci. Mater. Med. 2014, 25, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Huang, S.; Hou, Z.; Cheng, Z.; Yang, P.; Peng, C.; Lin, J. Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery. Biomaterials 2010, 31, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.R.; Venkatesh, R. A study of hydroxyapatite fibers prepared via sol–gel route. Mater Lett. 2004, 58, 3320–3323. [Google Scholar] [CrossRef]
- Franco, P.Q.; João, C.F.C.; Silva, J.C.; Borges, J.P. Electrospun hydroxyapatite fibers from a simple sol–gel system. Mater. Lett. 2012, 67, 233–236. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Hsu, H.W.; Chang, Y.H.; Yu, J.L.; Rau, L.R.; Tsai, S.W. Macroporous microbeads containing apatite-modified mesoporous bioactive glass nanofibres for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Huang, S.S.; Yu, W.X.; Hsu, Y.W.; Hsu, F.Y. Fabrication and characteristics of porous hydroxyapatite-CaO composite nanofibers for biomedical applications. Nanomaterials (Basel) 2018, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, C.M.; Pizziconi, V.; Alford, T.L.; Laursen, T. Hydroxyapatite powders and thin films prepared by a sol–gel technique. Thin Sol. Film. 1998, 326, 227–232. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Kuriakose, T.A.; Kalkura, S.N.; Palanichamy, M.; Arivuoli, D.; Dierks, K.; Bocelli, G.; Betzel, C. Synthesis of stoichiometric nano crystalline hydroxyapatite by ethanol-based sol–gel technique at low temperature. J. Cryst. Growth. 2004, 263, 517–523. [Google Scholar] [CrossRef]
- Kanchana, P.; Sekar, C. Influence of strontium on the synthesis and surface properties of biphasic calcium phosphate (BCP) bioceramics. J. Appl. Biomater. Biomech. 2010, 8, 153–158. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Weng, R.C.; Lin, H.M.; Lin, Y.H.; Lu, M.R.; Yu, J.L.; Hsu, H.W. A biomimetic extracellular matrix composed of mesoporous bioactive glass as a bone graft material. Microporous Mesoporous Mater. 2015, 212, 56–65. [Google Scholar] [CrossRef]
- Tsai, S.W.; Huang, C.C.; Rau, L.R.; Hsu, F.Y. Fabrication of aligned carbon nanotube/polycaprolactone/gelatin nanofibrous matrices for Schwann cell immobilization. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Liu, P.Y.; Wei, L.; Zou, Z.Y.; Zhang, W.B.; Qian, Y.; Shen, Y.H.; Chang, J. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. 2013, 222, 49–59. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Fredholm, Y.; de Rouffignac, A.; Hill, R.G. Structural analysis of a series of strontium-substituted apatites. Acta Biomater. 2008, 4, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Baddiel, C.B.; Berry, E.E. Spectra structure correlations in hydroxy and fluorapatite. Spectrochim. Acta 1966, 22, 1407–1416. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Ma, X.; Jiang, S.; Huang, Y.; Zhai, L.; Jiang, S. Preparation, characterization of electrospun meso-hydroxylapatite nanofibers and their sorptions on Co(II). J. Hazard. Mater. 2014, 265, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, A.; Takase, H.; Kandori, K.; Ishikawa, T. Preparation of calcium hydroxyapatite using amides. Polyhedron 1994, 13, 3071–3078. [Google Scholar] [CrossRef]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Karthikeyann, P.; Kavitha, L. Strontium, cerium co-substituted hydroxyapatite nanoparticles: Synthesis, characterization, antibacterial activity towards prokaryotic strains and in vitro studies. Coll. Surface A Physicochem. Eng. Asp. 2014, 451, 172–180. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Hatzistavrou, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Kontonasaki, E.; Boccaccini, A.R.; Paraskevopoulos, K.M. Characterization of the bioactive behaviour of sol–gel hydroxyapatite-CaO and Hydroxyapatite-CaO-bioactive glass composites. Mater. Sci. Eng. C 2010, 30, 497–502. [Google Scholar] [CrossRef]
- Ohyama, T.; Cowan, J.A. Calorimetric studies of metal binding to tetracycline. Role of solvent structure in defining the selectivity of metal ion-drug interactions. Inorg. Chem. 1995, 34, 3083–3086. [Google Scholar] [CrossRef]
- Rizzoli, R.; Reginster, J.Y.; Boonen, S.; Bréart, G.; Diez-Perez, A.; Felsenberg, D.; Kaufman, J.M.; Kanis, J.A.; Cooper, C. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif. Tissue Int. 2011, 89, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Doluisio, J.T.; Martin, A.N. Metal Complexation of the tetracycline hydrochlorides. J. Med. Chem. 1963, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bjørn, L.; Jostein, G. Reduction in the antibacterial effect of oxytetracycline in sea water by complex formation of magnesium and calcium. Dis. Aquat. Org. 1990, 9, 67–72. [Google Scholar]


 : CaO;
: CaO;  : HAp/Sr-HAP;
: HAp/Sr-HAP;  : CaCO3).
: CaCO3).
 : CaO;
: CaO;  : HAp/Sr-HAP;
: HAp/Sr-HAP;  : CaCO3).
: CaCO3).





| Sample Notation | Molar Ratio (Sr/Ca) | Molar Ratio (Sr + Ca)/P |
|---|---|---|
| 0mSrHANFs | 0 | 1.67 |
| 1mSrHANFs | 1/9 | 1.67 |
| 2mSrHANFs | 2/8 | 1.67 |
| 3mSrHANFs | 3/7 | 1.67 |
| Sample Name | Nominal Sr/(Sr + Ca) (%) | Measured Sr/(Sr + Ca) (%) | Nominal (Sr + Ca)/P | Measured (Sr + Ca)/P |
|---|---|---|---|---|
| 0mSrHANFs | 0 | 0 | 1.67 | 3.17 |
| 1mSrHANFs | 10 | 9.25 | 1.67 | 2.08 |
| 2mSrHANFs | 20 | 18.94 | 1.67 | 1.73 |
| 3mSrHANFs | 30 | 29.4 | 1.67 | 1.68 |
| Sample Name | Lattice Constants | d(002) (Å) | d(211) (Å) | |
|---|---|---|---|---|
| a (Å) | c (Å) | |||
| 0mSrHANFs | 9.72 | 7.02 | 3.51 | 2.90 |
| 1mSrHANFs | 9.80 | 7.06 | 3.53 | 2.92 |
| 2mSrHANFs | 9.83 | 7.08 | 3.54 | 2.93 |
| 3mSrHANFs | 9.90 | 7.12 | 3.56 | 2.95 |
| Sample Name | Ca10-xSrx(PO4)6(OH)2 (%) | CaO (%) | CaCO3 (%) |
|---|---|---|---|
| 0mSrHANFs | 66.3 | 21.1 | 12.6 |
| 1mSrHANFs | 77.5 | 21.5 | 1.0 |
| 2mSrHANFs | 83.4 | 15.8 | 0.8 |
| 3mSrHANFs | 96.1 | 1.4 | 2.5 |
| Sample Name | Surface Area (m2/g) | BJH Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| 0mSrHANFs | 8.62 | 0.065 | 21.8 |
| 1mSrHANFs | 8.41 | 0.043 | 21.2 |
| 2mSrHANFs | 7.87 | 0.052 | 25.1 |
| 3mSrHANFs | 8.11 | 0.053 | 25.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-W.; Yu, W.-X.; Hwang, P.-A.; Huang, S.-S.; Lin, H.-M.; Hsu, Y.-W.; Hsu, F.-Y. Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO3 Nanofibers with a Mesoporous Structure as Drug Delivery Carriers. Pharmaceutics 2018, 10, 179. https://doi.org/10.3390/pharmaceutics10040179
Tsai S-W, Yu W-X, Hwang P-A, Huang S-S, Lin H-M, Hsu Y-W, Hsu F-Y. Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO3 Nanofibers with a Mesoporous Structure as Drug Delivery Carriers. Pharmaceutics. 2018; 10(4):179. https://doi.org/10.3390/pharmaceutics10040179
Chicago/Turabian StyleTsai, Shiao-Wen, Wen-Xin Yu, Pai-An Hwang, Sheng-Siang Huang, Hsiu-Mei Lin, Yu-Wei Hsu, and Fu-Yin Hsu. 2018. "Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO3 Nanofibers with a Mesoporous Structure as Drug Delivery Carriers" Pharmaceutics 10, no. 4: 179. https://doi.org/10.3390/pharmaceutics10040179
APA StyleTsai, S. -W., Yu, W. -X., Hwang, P. -A., Huang, S. -S., Lin, H. -M., Hsu, Y. -W., & Hsu, F. -Y. (2018). Fabrication and Characterization of Strontium-Substituted Hydroxyapatite-CaO-CaCO3 Nanofibers with a Mesoporous Structure as Drug Delivery Carriers. Pharmaceutics, 10(4), 179. https://doi.org/10.3390/pharmaceutics10040179