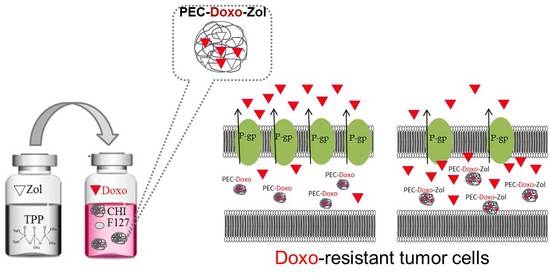
Chitosan-Based Polyelectrolyte Complexes for Doxorubicin and Zoledronic Acid Combined Therapy to Overcome Multidrug Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of Polyelectrolyte Complexes (PECs)
2.3. Size, Polydispersity Index (PI) and ζ Potential
2.4. Doxo and Zol Encapsulation Efficiency and Yield of the PECs
2.5. Cell Culture
2.6. Cell Proliferation Assay
2.7. Evaluation of Synergism
3. Results and Discussion
3.1. PECs Preparation and Characterization
3.2. Preparation and Characterization of PECs Encapsulating Doxo and Zol
3.3. Stability Studies
3.4. Cell Proliferation Assay
3.5. Evaluation of Synergism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PECs | polyelectrolyte complexes |
| Doxo | doxorubicin |
| Zol | zoledronic acid |
| MDR | multidrug resistance |
| ABC | ATP-binding cassette family |
| P-gp | P-glycoprotein |
| CHI | chitosan |
| TPP | sodium tripolyphosphate |
| SAOS | wild-type human osteosarcoma cells |
| SAOS DX | Doxo-resistant human osteosarcoma cells |
| U-2 OS | wild-type human bone osteosarcoma epithelial cells |
| U2-OS DX | Doxo-resistant human bone osteosarcoma epithelial cells |
| GPC | gel permeation chromatography |
| PEO | polyethylene oxide |
| PPO | polypropylene oxide |
| PI | polydispersity index |
| EE | encapsulation efficiency |
| UHPLC | ultra-high-performance liquid chromatography |
| IC50 | concentration inhibiting 50% of cell growth |
| CIs | indexes of combination |
| Q | precipitation flow rate |
References
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Liscovitch, M.; Lavie, Y. Cancer multidrug resistance: A review of recent drug discovery research. IDrugs 2002, 5, 349–355. [Google Scholar] [PubMed]
- Stavrovskaya, A.A. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Moscow) 2000, 65, 95–106. [Google Scholar] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.M.; Ambudkar, S.V. The molecular mechanisms of drug resistance in single-step and multi-step drug-selected cancer cells. Methods Mol. Biol. 2010, 596, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. The P-Glycoprotein Efflux Pump: How Does it Transport Drugs? J. Membr. Biol. 1997, 160, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimpl, G.; Burger, K.; Fahrenholz, F. Cholesterol as modulator of receptor function. Biochemistry 1997, 36, 10959–10974. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Mechanism and strategies to overcame multiple drug resistance in cancer. FEBS Lett. 2006, 580, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Troost, J.; Lindenmaie, J.; Haefeli, W.E.; Weiss, J. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol. Pharmacol. 2004, 66, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Caraglia, M.; Marra, M.; Naviglio, S.; Botti, G.; Addeo, R.; Abbruzzese, A. Zoledronic acid: An unending tale for an antiresorptive agent. Expert Opin. Pharmacother. 2010, 11, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Porto, S.; Lusa, S.; Gazzano, E.; Salzano, G.; Giordano, A.; Desiderio, V.; Ghigo, D.; Caraglia, M.; De Rosa, G.; et al. Self-assembling nanoparticles encapsulating zoledronic acid revert multidrug resistance in cancer cells. Oncotarget 2015, 6, 31461–31478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopecka, J.; Porto, S.; Lusa, S.; Gazzano, E.; Salzano, G.; Pinzòn-Daza, M.L.; Giordano, A.; Desiderio, V.; Ghigo, D.; De Rosa, G.; et al. Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin: A combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. Oncotarget 2016, 7, 20753–20772. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Salzano, G.; Leonetti, C.; Tassone, P.; Scarsella, M.; Zappavigna, S.; Calimeri, T.; Franco, R.; Liguori, G.; Cigliana, G.; et al. Nanotechnologies to use bisphosphonates as potent anticancer agents: The effects of zoledronic acid encapsulated into liposomes. Nanomed.: Nano. Biol. Med. 2011, 7, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Marra, M.; Porru, M.; Zappavigna, S.; Abbruzzese, A.; La Rotonda, M.I.; Leonetti, C.; Caraglia, M.; De Rosa, G. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int. J. Pharm. (Amsterdam, Neth.) 2011, 403, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Patwekar, S.L.; Potulwar, A.P.; Pedewad, S.R.; Gaikwad, M.S.; Khan, S.A.; Suryawanshi, A.B. Review on polyelectrolyte complex as novel approach for drug delivery system. IJPPR 2016, 5, 97–109. [Google Scholar]
- Mayol, L.; De Stefano, D.; Campani, V.; De Falco, F.; Ferrari, E.; Cencetti, C.; Matricardi, P.; Maiuri, L.; Carnuccio, R.; Gallo, A.; et al. Design and characterization of a chitosan physical gel promoting wound healing in mice. J. Mater. Sci.: Mater. Med. 2004, 25, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; Leonardis, P.D.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Tran, T.H.; Cho, H.J.; Kim, J.H.; Kim, Y.I.; Jeon, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Chitosan-Based Polyelectrolyte Complexes as Potential Nanoparticulate Carriers: Physicochemical and Biological Characterization. Pharm. Res. 2014, 31, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Scotlandi, K.; Manara, M.C.; Maurici, D.; Lollini, P.L.; De Giovanni, C.; Toffoli, G.; Baldini, N. Establishment and characterization of multidrug-resistant human osteosarcoma cell lines. Anticancer Res. 1993, 13, 323–329. [Google Scholar] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Controlled Release 2001, 73, 255–261. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Atyabi, F.; Dinarvand, R.; Ostad, S.N. Chitosan–Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar] [CrossRef]
- Alberts, D.S.; Garcia, D.J. Safety aspects of pegylated liposomal doxorubicin in patients with cancer. Drugs 1997, 4, 30–35. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997, 4, 15–21. [Google Scholar] [CrossRef]
- Working, P.K.; Newman, M.S.; Sullivan, T.; Yarrington, J. Reduction of the cardiotoxicity of doxorubicin in rabbits and dogs by encapsulation in long-circulating, pegylated liposomes. J. Pharmacol. Exp. Ther. 1999, 289, 1128–1133. [Google Scholar] [PubMed]
- Zeng, X.; Morgenstern, R.; Nyström, A.M. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-Mediated drug resistance. Biomaterials 2014, 35, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the Use of PEGylation for Drug Delivery. Drug Delivery Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef]



| Formulation | (CHI) mg/mL | (TPP) mg/mL | D (nm) | PI |
|---|---|---|---|---|
| PEC(CHI 0.4-TPP 0.5) | 0.4 | 0.5 | 236.9 ± 31.2 | 1.14 ± 0.5 |
| PEC(CHI 0.3-TPP 0.5) | 0.3 | 0.5 | 231.9 ± 9.50 | 0.23 ± 0.1 |
| PEC(CHI 0.5-TPP 0.4) | 0.5 | 0.4 | 272.1 ± 86.7 | 0.42 ± 0.1 |
| PEC(CHI 0.5-TPP 0.3) | 0.5 | 0.3 | 314.2 ± 14.5 | 1.03 ± 0.6 |
| PEC(CHI 0.5-TPP 0.5) | 0.5 | 0.5 | 132.7 ± 11.2 | 0.43 ± 0.1 |
| PEC(CHI 0.4-TPP 0.4) | 0.4 | 0.4 | 185.1 ± 0.81 | 0.27 ± 0.1 |
| PEC(CHI 0.3-TPP 0.3) | 0.3 | 0.3 | 132.3 ± 6.80 | 0.42 ± 0.1 |
| PEC(CHI 0.3-TPP 0.5) | 0.3 | 0.4 | 287.8 ± 15.4 | 0.15 ± 0.1 |
| Formulation | (CHI) mg/mL | (TPP) mg/mL | Q (µL/min) | d (nm) | PI | ζ Potential (mV) |
|---|---|---|---|---|---|---|
| PEC(CHI 0.5-TPP 0.5)-A | 0.5 | 0.5 | 500 | 223.3 ± 3.8 | 0.67 ± 0.3 | 18.1 ± 1.5 |
| PEC(CHI 0.5-TPP 0.5)-B | 0.5 | 0.5 | 133.3 | 187.0 ± 9.1 | 0.45 ± 0.2 | 21.3 ± 1.1 |
| PEC(CHI 0.4-TPP 0.4)-A | 0.4 | 0.4 | 500 | 147.7 ± 2.9 | 0.49 ± 0.1 | 19.1 ± 1.9 |
| PEC(CHI 0.4-TPP 0.4)-B | 0.4 | 0.4 | 133.3 | 129.4 ± 2.7 | 0.42 ± 2.7 | 19.1 ± 0.8 |
| PEC(CHI 0.3-TPP 0.3)-A | 0.3 | 0.3 | 500 | 127.5 ± 2.2 | 0.51 ± 0.3 | 17.8 ± 2.9 |
| PEC(CHI 0.3-TPP 0.3)-B | 0.3 | 0.3 | 133.3 | 104.4 ± 1.4 | 0.41 ± 0.3 | 21.4 ± 1.9 |
| Formulation | (CHI) mg/mL | (TPP) mg/mL | F127 (% w/w) | d (nm) | PI | ζ Potential (mV) |
|---|---|---|---|---|---|---|
| PEC(CHI 0.3-TPP 0.3)-B20 | 0.3 | 0.3 | 20 | 114.1 ± 5.1 | 0.48 ± 0.1 | 17.2 ± 2.5 |
| PEC(CHI 0.3-TPP 0.3)-B16 | 0.3 | 0.3 | 16 | 114.8 ± 6.9 | 0.32 ± 0.2 | 16.9 ± 2.1 |
| PEC(CHI 0.3-TPP 0.3)-B10 | 0.3 | 0.3 | 10 | 110.8 ± 3.5 | 0.23 ± 0.2 | 21.2 ± 3.3 |
| Formulation | (Zol)-Loaded (mg/mL) | (Doxo)-Loaded (mg/mL) | EE Zol (%) | EE Doxo (%) | Yield (%) |
|---|---|---|---|---|---|
| PEC | - | - | - | - | 80.8 ± 0.1 |
| PEC-Zol | 0.8 | - | 92.3 ± 5.3 | - | 77.8 ± 1.3 |
| PEC-Doxo | - | 0.4 | - | 25.8 ± 0.5 | 79.4 ± 0.1 |
| PEC-Doxo-Zol | 0.8 | 0.4 | 83.1 ± 11 | 29.2 ± 6.6 | 81.6 ± 0.1 |
| Formulation | d (nm) | PI | ζ Potential (mV) |
|---|---|---|---|
| PEC-Zol | 131.1 ± 0.1 | 0.32 ± 0.1 | 20.2 ± 1.3 |
| PEC-Doxo | 120.5 ± 4.7 | 0.42 ± 0.4 | 22.9 ± 1.6 |
| PEC-Doxo-Zol | 111.5 ± 0.1 | 0.39 ± 0.4 | 23.1 ± 2.3 |
| SAOS | IC50Zol | SAOS | IC50Doxo |
| Zol | 17 | Doxo | 2 |
| PEC-Zol | 16 | PEC-Doxo | 0.5 |
| PEC-Doxo-Zol | 0.8 | PEC-Doxo-Zol | 0.05 |
| PEC | - | PEC | - |
| SAOS DX | IC50Zol | SAOS DX | IC50Doxo |
| Zol | 23.4 | Doxo | >20 |
| PEC-Zol | >200 | PEC-Doxo | 10.4 |
| PEC-Doxo-Zol | 13.8 | PEC-Doxo-Zol | 0.9 |
| PEC | - | PEC | - |
| U-2 OS | IC50Zol | U-2 OS | IC50Doxo |
| Zol | 15.60 | Doxo | 0.14 |
| PEC-Zol | 40.40 | PEC-Doxo | 0.06 |
| PEC-Doxo-Zol | <0.78 | PEC-Doxo-Zol | <0.05 |
| PEC | - | PEC | - |
| U-2 OS DX | IC50Zol | U-2 OS DX | IC50Doxo |
| Zol | >100 | Doxo | >6.60 |
| PEC-Zol | >100 | PEC-Doxo | >6.60 |
| PEC-Doxo-Zol | 13.60 | PEC-Doxo-Zol | 0.80 |
| PEC | - | PEC | - |
| Cell Lines | CI50 | Interpretation |
|---|---|---|
| SAOS | 0.3 | Strong Synergism |
| SAOS DX | 0.4 | Strong Synergism |
| U-2 OS | 0.7 | Synergism |
| U-2 OS DX | 0.3 | Strong Synergism |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarra, S.; Zappavigna, S.; Campani, V.; Abate, M.; Cossu, A.M.; Leonetti, C.; Porru, M.; Mayol, L.; Caraglia, M.; De Rosa, G. Chitosan-Based Polyelectrolyte Complexes for Doxorubicin and Zoledronic Acid Combined Therapy to Overcome Multidrug Resistance. Pharmaceutics 2018, 10, 180. https://doi.org/10.3390/pharmaceutics10040180
Giarra S, Zappavigna S, Campani V, Abate M, Cossu AM, Leonetti C, Porru M, Mayol L, Caraglia M, De Rosa G. Chitosan-Based Polyelectrolyte Complexes for Doxorubicin and Zoledronic Acid Combined Therapy to Overcome Multidrug Resistance. Pharmaceutics. 2018; 10(4):180. https://doi.org/10.3390/pharmaceutics10040180
Chicago/Turabian StyleGiarra, Simona, Silvia Zappavigna, Virginia Campani, Marianna Abate, Alessia Maria Cossu, Carlo Leonetti, Manuela Porru, Laura Mayol, Michele Caraglia, and Giuseppe De Rosa. 2018. "Chitosan-Based Polyelectrolyte Complexes for Doxorubicin and Zoledronic Acid Combined Therapy to Overcome Multidrug Resistance" Pharmaceutics 10, no. 4: 180. https://doi.org/10.3390/pharmaceutics10040180
APA StyleGiarra, S., Zappavigna, S., Campani, V., Abate, M., Cossu, A. M., Leonetti, C., Porru, M., Mayol, L., Caraglia, M., & De Rosa, G. (2018). Chitosan-Based Polyelectrolyte Complexes for Doxorubicin and Zoledronic Acid Combined Therapy to Overcome Multidrug Resistance. Pharmaceutics, 10(4), 180. https://doi.org/10.3390/pharmaceutics10040180