Zwitterionic Cysteine Drug Coating Influence in Functionalization of Implantable Ti50Zr Alloy for Antibacterial, Biocompatibility and Stability Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Substrate Coating Protocol
2.3. Surface Characterizations
2.4. Electrochemical Tests
2.5. In Vitro Biocompatibility Assessment
2.6. Antibacterial Effect
3. Results and Discussions
3.1. Surface Characterization
3.2. Electrochemical Tests
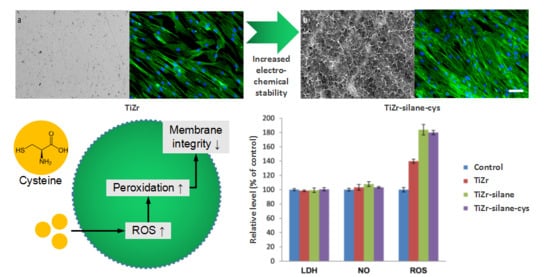
3.3. In Vitro Biocompatibility
3.4. Antibacterial Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. J. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr blloy improve biological response. ActaBiomater 2015, 24, 370–377. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of Vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Galdos, M.V.; Pastore, J.I.; Ballarre, J.; Ceré, S.M. Dual-surface modification of titanium alloy with anodizing treatment and bioceramic particles for enhancing prosthetic devices. J. Mater. Sci. 2017, 52, 9151–9165. [Google Scholar] [CrossRef] [Green Version]
- Vasilescu, C.; Calderon Moreno, J.M.; Drob, S.I.; Popa, M. Influence of thermo-mechanical processing on the microstructure, mechanical properties and corrosion behaviour of a new quaternary titanium alloy. Mater. Corros. 2014, 65, 715–724. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Liedberg, B. L-Cysteine adsorbed on gold and copper: An X-ray photoelectron spectroscopy study. J. Colloid Interface Sci. 1992, 149, 162–173. [Google Scholar] [CrossRef]
- Mindroiu, M.; Cicek, E.; Miculescu, F.; Demetrescu, I. The influence of thermal oxidation treatment on the electrochemical stability of TiAlV and TiAlFe alloys and their potential application as biomaterials. Rev. Chim. (Bucharest) 2007, 58, 898–903. [Google Scholar]
- Grandin, H.M.; Berner, S.; Dard, M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Chelariu, R.; Mareci, D.; Munteanu, C. Preliminary electrochemical testing of some Zr–Ti alloys in 0.9% NaCl solution. Mater. Corros. 2013, 64, 585–591. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. The corrosion resistance of Ti-Zr binary alloy with compositional change. In Frontiers in Bioengineering and Biotechnology, Conference Abstract: 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016; Curran Associates, Inc.: Red Hook, NY, USA, 2016. [Google Scholar] [CrossRef]
- Brault, N.D.; Sundaram, H.S.; Li, Y.; Huang, C.J.; Yu, Q.; Jiang, S. Dry film refractive index as an important parameter for ultra-low fouling surface coatings. Biomacromolecules 2012, 13, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xue, H.; Carr, L.R.; Wang, J.; Jiang, S. Zwitterionic Poly(Carboxybetaine) hydrogels for glucose biosensors in complex media. Biosens. Bioelectron. 2011, 26, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. Fda-approved Poly(Ethylene Glycol)–protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Chen, S.; Ma, C.; Zhang, G. Biodegradable polymer as controlled release system of organic antifoulant to prevent marine biofouling. Prog. Org. Coat. 2017, 104, 58–63. [Google Scholar] [CrossRef]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O., 3rd. Sustained release drug delivery applications of polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of structure−property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Lin, P. Improving Biocompatibility of Implantable Bioelectronics Using Zwitterionic Cysteine. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2017. [Google Scholar]
- Lin, P.; Lin, C.W.; Mansour, R.; Gu, F. Improving biocompatibility by surface modification techniques on implantable bioelectronics. Biosens. Bioelectron. 2013, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.; Wirsén, A.; Norberg, Å.; Frie, J.; Printz, G.; Lagercrantz, H.; Gudmundsson, G.H.; Agerberth, B.; Jonsson, B. A novel cysteine-linked antibacterial surface coating significantly inhibits bacterial colonization of nasal silicone prongs in a phase one pre-clinical trials. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, Histidine and Glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.E.; Gu, F.X. Surface Functionalization of silica nanoparticles with Cysteine: A low-fouling zwitterionic surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Han, H. One-step synthesis of Cystine-coated gold nanoparticles in aqueous solution. Colloids Surf. A 2008, 317, 229–233. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Vazquez, F.J.; Perez, M.; Santamaria, J.; Arruebo, M.; Lujan, L. Porous orthopedic steel implant as an antibiotic eluting device: Prevention of post-surgical infection on an ovine model. Int. J. Pharm. 2013, 452, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing antimicrobial activity of TiO2/Ti by Torularhodinbioinspired surface modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Indira, K.; KamachiMudali, U.; Rajendran, N. In vitro bioactivity and corrosion resistance of Zr incorporated TiO2 nanotube arrays for orthopaedic applications. Appl. Surf. Sci. 2014, 316, 264–275. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Li, Z.; Wu, Z.; Li, S. The improvement in cryogenic mechanical properties of Nano-ZrO2/Epoxy composites via surface modification of Nano-ZrO2. RSC Adv. 2016, 6, 61393–61401. [Google Scholar] [CrossRef]
- Toiserkani, H. Fabrication and characterization chitosan/functionalized zinc oxide bionanocomposites and study of their antibacterial activity. Compos. Interface 2016, 23, 175–189. [Google Scholar] [CrossRef]
- Karaoglu, E.; Summak, M.M.; Baykal, A.; Sozeri, H.; Toprak, M.S. Synthesis and characterization of catalytically activity Fe3O4–3-Aminopropyl-Triethoxysilane/Pd nanocomposite. J. Inorg. Organomet. Polym. 2013, 23, 409–417. [Google Scholar] [CrossRef]
- Gray-Munro, J. Biomimetic Surface Modifications of Magnesium and Magnesium Alloys for Biomedical Applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Narayanan, T.S.N.S., Park, I., Lee, M., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2015; pp. 271–299. [Google Scholar]
- Zu, Y.; Zhao, Q.; Zhao, X.; Zu, S.; Meng, L. Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method. Int. J. Nanomed. 2011, 6, 3429–3441. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liao, L.; Ding, Y.; Zeng, H. Dithizone-etched CdTe nanoparticles-based fluorescence sensor for the off–on detection of cadmium ion in aqueous media. RSC Adv. 2017, 7, 10361–10368. [Google Scholar] [CrossRef] [Green Version]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and chromium adsorption from water using L-Cysteine functionalized magnetite (Fe3O4) nanoparticles. Sci. Rep. 2017, 7, 7672. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, L.; Tsao, H.K.; Sheng, Y.J.; Chen, S.; Jiang, S. Strong repulsive forces between protein and oligo (Ethylene Glycol) self-assembled monolayers: A molecular simulation study. Biophys. J. 2005, 89, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.N.; Chang, S.H.; Park, S.J.; Lim, J.; Lee, J.; Yoon, T.J.; Kim, J.S.; Cho, M.H. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS ONE 2015, 10, e0131208. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, M.; Krajnović, T.; Maksimović-Ivanić, D.; Graf, H.L.; Mijatović, S. Ti-Slactive and TiZr-Slactive dental implant surfaces promote fast osteoblast differentiation. Coatings 2017, 7, 102. [Google Scholar] [CrossRef]
- Ujihara, Y.; Nakamura, M.; Miyazaki, H.; Wada, S. Contribution of actin filaments to the global compressive properties of fibroblasts. J. Mech. Behav. Biomed. Mater. 2012, 14, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Structure and Organization of actin filaments. In The Cell: A Molecular Approach, 2nd ed.; Cooper, G.M., Ed.; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN 10 0-87893-106-6. [Google Scholar]
- Harris, C.L. Cysteine and growth inhibition of escherichia coli: threonine deaminase as the target enzyme. J. Bacteriol. 1981, 145, 1031–1035. [Google Scholar] [PubMed]
- Kari, C.; Nagy, Z.; Kovacs, P.; Hernadi, F. Mechanism of the growth inhibitory effect of cysteine on Escherichia Coli. J. Gen. Microbiol. 1971, 68, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.F.; Montville, T.; Blais, K. Toxic Effect of Cysteine against Salmonella Typhimurium. Appl. Environ. Microbiol. 1980, 39, 1081–1083. [Google Scholar] [PubMed]
- Brooks, G.F.; Carroll, K.C.; Butel, J.S.; Morse, S.A.; Mietzner, T.A. The Staphylococci. In Jawetz, Melnick & Adelberg’s Medical Microbiology, 23rd ed.; Brooks, G.F., Carroll, K.C., Butel, J.S., Morse, S.A., Mietzner, T.A., Eds.; McGraw-Hill Companies: New York, NY, USA, 2004; Volume 233, pp. 225–227. [Google Scholar]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The Role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, F.; Boyd, D.; Beckwith, J. Disulfide Bond formation in prokaryotes: History, diversity and design. Biochim. Biophys. Acta 2014, 1844, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J. Laboratory Methods for studies of bacterial adhesion. J. Microbiol. Meth. 1997, 30, 141–152. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]









| Immersion Time (h) | Sample | Ecorr (V) | Jcorr (A/cm2) | Corrosion Rate (mm/year) |
|---|---|---|---|---|
| 0 | Ti50Zr | −0.504 | 1.361 × 10−6 | 131.1 × 10−4 |
| Ti50Zr-cys | −0.417 | 4.048 × 10−8 | 3.898 × 10−4 | |
| 24 | Ti50Zr | −0.413 | 3.712 × 10−8 | 3.574 × 10−4 |
| Ti50Zr-cys | −0.198 | 1.401 × 10−8 | 1.349 × 10−4 | |
| 48 | Ti50Zr | −0.374 | 3.481 × 10−8 | 3.322 × 10−4 |
| Ti50Zr-cys | −0.144 | 1.450 × 10−8 | 1.396 × 10−4 | |
| 72 | Ti50Zr | −0.366 | 3.182 × 10−8 | 3.064 × 10−4 |
| Ti50Zr-cys | −0.105 | 1.400 × 10−8 | 1.348 × 10−4 |
| Immersion Time (h) | Sample | Rs (Ω) | R2 (Ω) ×10−5 | CPE2 | R1 (Ω) ×10−6 | CPE1 | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Yo1 (S × sn) ×105 | N2 | Yo2 (S × sn) ×105 | N1 | ||||||
| 0 | Ti50Zr | 63.79 | - | - | - | 0.01 | 5.22 | 0.89 | 0.05 |
| Ti50Zr-cys | 52.74 | 1.23 | 3.48 | 0.91 | 1.40 | 1.18 | 0.83 | 0.01 | |
| 24 | Ti50Zr | 58.73 | - | - | - | 1.32 | 2.47 | 0.92 | 0.02 |
| Ti50Zr-cys | 66.87 | 1.64 | 3.06 | 0.92 | 3.77 | 1.42 | 0.85 | 0.01 | |
| 48 | Ti50Zr | 61.53 | - | - | - | 1.49 | 2.23 | 0.92 | 0.02 |
| Ti50Zr-cys | 60.92 | 1.40 | 2.87 | 0.93 | 4.09 | 1.18 | 0.89 | 0.007 | |
| 72 | Ti50Zr | 61.62 | - | - | - | 1.59 | 2.08 | 0.92 | 0.02 |
| Ti50Zr-cys | 59.03 | 1.10 | 2.61 | 0.94 | 4.45 | 1.05 | 0.90 | 0.004 | |
| Sample | Ti50Zr | TiZr-cys |
|---|---|---|
| I% S. aureus | 27.39 ± 0.15 | 56.74 ± 0.28 |
| I% E. coli | 29.15 ± 0.23 | 63.90 ± 0.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demetrescu, I.; Dumitriu, C.; Totea, G.; Nica, C.I.; Dinischiotu, A.; Ionita, D. Zwitterionic Cysteine Drug Coating Influence in Functionalization of Implantable Ti50Zr Alloy for Antibacterial, Biocompatibility and Stability Properties. Pharmaceutics 2018, 10, 220. https://doi.org/10.3390/pharmaceutics10040220
Demetrescu I, Dumitriu C, Totea G, Nica CI, Dinischiotu A, Ionita D. Zwitterionic Cysteine Drug Coating Influence in Functionalization of Implantable Ti50Zr Alloy for Antibacterial, Biocompatibility and Stability Properties. Pharmaceutics. 2018; 10(4):220. https://doi.org/10.3390/pharmaceutics10040220
Chicago/Turabian StyleDemetrescu, Ioana, Cristina Dumitriu, Georgeta Totea, Cristina I. Nica, Anca Dinischiotu, and Daniela Ionita. 2018. "Zwitterionic Cysteine Drug Coating Influence in Functionalization of Implantable Ti50Zr Alloy for Antibacterial, Biocompatibility and Stability Properties" Pharmaceutics 10, no. 4: 220. https://doi.org/10.3390/pharmaceutics10040220
APA StyleDemetrescu, I., Dumitriu, C., Totea, G., Nica, C. I., Dinischiotu, A., & Ionita, D. (2018). Zwitterionic Cysteine Drug Coating Influence in Functionalization of Implantable Ti50Zr Alloy for Antibacterial, Biocompatibility and Stability Properties. Pharmaceutics, 10(4), 220. https://doi.org/10.3390/pharmaceutics10040220