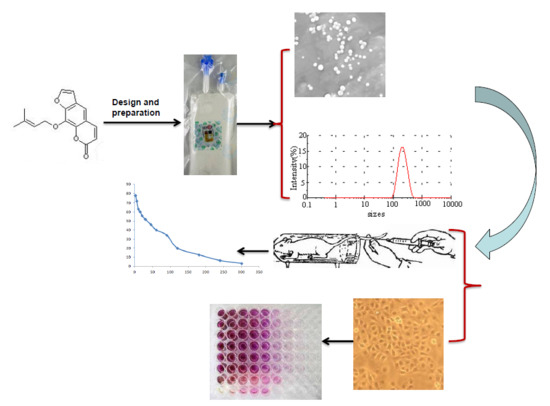
Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Drugs
2.1.2. Animals
2.2. Methods
2.2.1. Imperatorin Lipid Microsphere Preparation
2.2.2. Measurement of Size, PDI, and Zeta Potential of Imperatorin Lipid Microsphere
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Determination of Drug Loading and Encapsulation Efficiency
2.2.5. RSM Design and Optimization of Imperatorin Lipid Microsphere Preparation Conditions
2.2.6. Pharmacokinetics and Statistical Analysis
2.2.7. Effect of Imperatorin and Imperatorin Lipid Microspheres on MDA-MB-231 Cell Proliferation
2.3. Data Analysis
3. Results and Discussion
3.1. Central Composite Design of Response Surface Methodology
003BC − 0.096A2 − 0.097B2 − 0.035C2
003AC + 9.20457E − 003BC − 0.095557A2 − 0.096566B2 − 0.035043C2
3.2. Drug Loading and Encapsulation Efficiency
3.3. Particle Size and Zeta Potential Measurements
3.4. Scanning Electron Microscopy (SEM)
3.5. Pharmacokinetic Study
3.6. Effect of Imperatorin and Imperatorin Lipid Microspheres on MDA-MB-231 Cell Proliferation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koziol, E.; Skalicka-Wozniak, K. Imperatorin-pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Choochuay, K.; Chunhacha, P.; Pongrakhananon, V.; Luechapudiporn, R.; Chanvorachote, P. Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med. 2013, 67, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Sun, J.G.; Chan, J.Y.; Yang, L.; Wu, S.H.; Fung, K.P.; Liu, F.Y. Anticancer Effects of Imperatorin Isolated from Angelica dahurica: Induction of Apoptosis in HepG2 Cells through both Death-Receptor- and Mitochondria-Mediated Pathways. Chemotherapy 2011, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Shao, L.L.; Ruan, Y.P. Anti-tumor Effect and Mechanism of Imperatorin Enhances the Cytotoxicity of Cisplatin Osteosarcoma Cells. Chin. J. Mod. Appl. Pharm. 2015, 32, 1193–1197. [Google Scholar]
- Zheng, Y.; Jiang, K. Antitumor effect of imperatorin enhances cytotoxicity of doxorubicin to HeLa cells. Chin. J. Pathophysiol. 2015, 31, 1578–1583. [Google Scholar]
- Bqdziul, D.; Jakubowicz-Gil, J.; Paduch, R.; Glowniak, K.; Gawron, A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol. Cell. Biochem. 2014, 392, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowicz-Gi, J.; Paduch, R.; Ulz, Z.; Badziul, D.; Glowniak, K.; Gawron, A. Cell death in HeLa cells upon imperatorin and cisplatin treatment Cell death in HeLa cells upon imperatorin and cisplatin treatment. Folia Histochem. Cytochem. 2012, 50, 381–391. [Google Scholar] [CrossRef]
- Badziula, D.; Jakubowicz-Gila, J.; Lanqner, E.; Rzeski, W.; Glowniak, K.; Gawron, A. The effect of quercetin and imperatorin on programmed cell death induction in T98G cells in vitro. Pharmacol. Rep. 2014, 62, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Q.; Wang, L.; He, L. Develpoment and validation of a gas chromatography-mass spectrometry method for the determination of imperatorin in rat plasma and tissue: Application to study its pharmazokinetics. Anal. Sci. 2009, 25, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wei, X.X.; Zhou, M.N.; Zeng, L.; Shu, J.C.; Yang, M. Research progress in traditional Chinese medicine injectable emulsion. Chin. J. New Drugs 2015, 24, 1980–1984. [Google Scholar]
- Lin, H.; Xie, Q.; Huang, X.; Ban, J.; Wang, B. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.J.; Lu, W.; Li, C.H.; Wang, S.C.; He, L.C. Imperatorin sustained-release tablets: In vitro and pharmacokinetic studies. Arch. Pharm. Res. 2010, 33, 1209. [Google Scholar] [CrossRef] [PubMed]
- Norden, T.P.; Siekmann, B.; Lundquist, S.; Malmsten, M. Physicochemical characterisation of a rug-containing phospholipid-stabilised O/W emulsion for intravenous administration. Eur. J. Pharm. Sci. 2001, 13, 393–401. [Google Scholar] [CrossRef]
- Medina, J.; Salvado, A.; del Pozo, A. Use of ultrasound to prepare lipid emulsions of lorazepam for intravenous injection. Int. J. Pharm. 2001, 216, 1–8. [Google Scholar] [CrossRef]
- Auriemma, G.; Mencherini, T.; Russo, P. Prilling for the development of multi-particulate colon durg delivery systems: Pectin vs. pectin-alginate beads. Carbohydr. Polym. 2013, 92, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Illum, L. Colonic durg delivery. Drug Dev. Ind. Pharm. 1997, 23, 893–913. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Inqvarsson, P.T.; van de Weert, M.; Andersen, P.; Follmann, F. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J. Control. Release 2015, 210, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.T.; Paxton, J.; Lim, J.; Li, Y.; Wu, Z.M. Development of a gradient high performance liquid chromatography assay for simultaneous analysis of hydrophilic gemcitabine and lipophilic curcumin using a central composite design and its application in liposome development. J. Pharm. Biomed. 2014, 98, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Ghaffari, S.; Khoshayand, M.R. Development and optimization of solidlipid nanoparticles of amikacin by central composite design. J. Liposome Res. 2010, 20, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.W.; Li, X. Study on antitumor activity of five Furanocoumarins from the root bark of Changium smyrnioides in vitro. Chin. J. Exp. Tradit. Med. Formul. 2012, 18, 203–205. [Google Scholar]
- Chen, H. Apoptosis of human breast cancer cell line MCF-7 induced by orbixin. Zhejiang Pract. Med. 2015, 20, 177–179. [Google Scholar]
- Yang, X.W.; Xu, B.; Ran, F.X.; Wang, R.Q.; Wu, J.; Cui, J.R. Inhibitory effect of 40 Coumarins Compounds against growth of human epidermal carcinoma a cell line A432 and human mammary cancer cell line BCAP in vitro. Mod. Chin. Med. 2006, 8, 9–10. [Google Scholar]
- Wang, Y.; Tang, X.; Cai, C. Study of tacrolimus-loaded lipid microsphere preparation. Chin. J. Pharm. 2014, 12, 167–176. [Google Scholar]
- Liu, A.; Chen, H.; Tang, X. Preparation and physical stability of astragaloside IV lipid microspheres for injection. Chin. J. Pharm. 2009, 7, 290–298. [Google Scholar]
- Pharmacopoeia Commission of the Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Yang, S.Y.; Chen, J.Y.; Zhao, D.; Han, D.; Chen, X.J. Comparative study on preparative methods of DC-Chol/DOPE liposomes and formulation optimization by determining encapsulation efficiency. Int. J. Pharm. 2012, 434, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cui, G.H.; Lu, B. Optimization of multiple evariables: Application of central composite design and overall desirability. Chin. Pharm. J. 2000, 35, 530–533. [Google Scholar]




| Factors | Code | Range and Levels | ||||
|---|---|---|---|---|---|---|
| −1.732 | −1 | 0 | 1 | 1.732 | ||
| egg lecithin | A | 1 | 1.11 | 1.25 | 1.39 | 1.5 |
| Poloxamer 188 | B | 0.1 | 0.21 | 0.35 | 0.49 | 0.6 |
| Soybean oil/oil phase | C | 0 | 10.57 | 25.00 | 39.43 | 50 |
| No. Levels of Independent Factors’ Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | Y3 | Y4 | Y5 | OD | |
| 1 | 1.11 | 0.21 | 39.43 | 177 | 0.148 | −43.4 | 6.58 | 89% | 0 |
| 2 | 1.25 | 0.35 | 25.00 | 172 | 0.131 | −44.1 | 7.59 | 90% | 0.4835 |
| 3 | 1.11 | 0.49 | 10.57 | 169 | 0.168 | −47.0 | 8.27 | 89% | 0.5195 |
| 4 | 1.25 | 0.35 | 25.00 | 172 | 0.128 | −43.7 | 7.72 | 88% | 0.4582 |
| 5 | 1.25 | 0.35 | 25.00 | 161 | 0.138 | −45.0 | 7.29 | 89% | 0.4562 |
| 6 | 1.39 | 0.49 | 39.43 | 164 | 0.097 | −38.7 | 6.93 | 81% | 0 |
| 7 | 1.25 | 0.35 | 25.00 | 165 | 0.148 | −43.5 | 7.23 | 90% | 0.4013 |
| 8 | 1.25 | 0.35 | 0 | 167 | 0.129 | −43.4 | 7.16 | 84% | 0.3693 |
| 9 | 1.11 | 0.21 | 10.57 | 193 | 0.122 | −45.2 | 9.43 | 91% | 0 |
| 10 | 1.0 | 0.35 | 25.00 | 201 | 0.132 | −43.9 | 9.02 | 89% | 0 |
| 11 | 1.5 | 0.35 | 25.00 | 168 | 0.134 | −43.8 | 7.33 | 88% | 0.4629 |
| 12 | 1.39 | 0.49 | 39.43 | 177 | 0.183 | −41.9 | 9.14 | 89% | 0.5391 |
| 13 | 1.39 | 0.49 | 10.57 | 154 | 0.116 | −44.5 | 8.28 | 81% | 0 |
| 14 | 1.11 | 0.49 | 39.43 | 165 | 0.094 | −42.4 | 7.29 | 88% | 0.4037 |
| 15 | 1.39 | 0.21 | 10.57 | 168 | 0.138 | −43.5 | 8.33 | 90% | 0.7286 |
| 16 | 1.25 | 0.35 | 25.00 | 164 | 0.120 | −43.5 | 7.76 | 89% | 0.6020 |
| 17 | 1.25 | 0.35 | 50.00 | 176 | 0.136 | −44.7 | 7.88 | 90% | 0.4567 |
| 18 | 1.25 | 0.35 | 25.00 | 170 | 0.129 | −43.1 | 10.42 | 90% | 0.6491 |
| 19 | 1.25 | 0.1 | 25.00 | 196 | 0.097 | −41.5 | 10.92 | 92% | 0.4569 |
| 20 | 1.25 | 0.6 | 25.00 | 170 | 0.096 | −40.5 | 9.27 | 93% | 0 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > 7 |
|---|---|---|---|---|---|
| Model | 1.05 | 9 | 0.12 | 9.16 | 0.0009 * |
| A-A | 0.094 | 1 | 0.094 | 7.36 | 0.0218 |
| B-B | 0.092 | 1 | 0.092 | 7.23 | 0.0228 |
| C-C | 1.691 × 10−3 | 1 | 1.691 × 10−3 | 0.13 | 0.7233 |
| AB | 0.60 | 1 | 0.60 | 108.14 | <0.0001 |
| AC | 6.778 × 10−4 | 1 | 6.778 × 10−4 | 0.053 | 0.8223 |
| BC | 6.778 × 10−4 | 1 | 6.778 × 10−4 | 0.053 | 0.8223 |
| A2 | 0.14 | 1 | 0.14 | 11.28 | 0.0073 |
| B2 | 0.15 | 1 | 0.15 | 11.52 | 0.0068 |
| C2 | 0.019 | 1 | 0.019 | 1.52 | 0.2462 |
| Residual | 0.13 | 10 | 0.013 | ||
| Lack of Fit | 0.082 | 5 | 0.016 | 1.78 | 0.2713 |
| Pure Error | 0.046 | 5 | 9.174 × 10−3 | ||
| Cor Total | 1.18 | 19 |
| Item | Data | Item | Data |
|---|---|---|---|
| Std. Dev. | 0.11 | R-Squared | 0.8918 |
| Mean | 0.35 | Adj R-Squared | 0.7944 |
| C.V.% | 32.32 | Pred R-Square | 0.4031 |
| PRESS | 0.70 | Adeq Precision | 9.418 |
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Important |
|---|---|---|---|---|---|---|
| A: egg lecithin | is in range | 1.0 | 1.5 | 1 | 1 | 3 |
| B: poloxamer 188 | is in range | 0.1 | 0.6 | 1 | 1 | 3 |
| C: LCT/oil phase ratio | is in range | 0 | 50 | 1 | 1 | 3 |
| Responses: OD | maximize | 0 | 0.7286 | 1 | 1 | 3 |
| Batch | A | B | C | OD | ||
|---|---|---|---|---|---|---|
| Predicted Value | Experimental Value | Percent Prediction Error | ||||
| 20171101 | 1.39 | 0.21 | 10.57 | 0.7580 | 0.7286 | 3.8% |
| 20171102 | 1.39 | 0.21 | 10.57 | 0.7580 | 0.7395 | 2.4% |
| 20171103 | 1.39 | 0.21 | 10.57 | 0.7580 | 0.7251 | 4.3% |
| Batch | Drug Loading (mg/mL) | Encapsulation Efficiency (%) |
|---|---|---|
| 20171101 | 0.815 | 90.3 |
| 20171102 | 0.836 | 91.2 |
| 20171103 | 0.859 | 88.7 |
| Mean | 0.833 ± 0.027 | 90.0 ± 1.27 |
| Batch | Zeta Potential (mv) | Particle Size (nm) | PDI |
|---|---|---|---|
| Carrier | −44.9 ± 1.20 | 154 ± 4.92 | 0.157 ± 0.04 |
| 20171101 | −43.1 | 169 | 0.114 |
| 20171102 | −44.1 | 165 | 0.159 |
| 20171103 | −43.5 | 169 | 0.142 |
| Mean | −43.5 ± 0.50 | 168 ± 1.73 | 0.138 ± 0.02 |
| Parameter | Unit | Route of Administration | |
|---|---|---|---|
| Intravenous Injection | Oral Administration | ||
| AUC(0–t) | mg/L·h | 116.71 ± 38.72 ** | 15.92 ± 5.10 |
| AUC(0–∞) | mg/L·h | 121.24 ± 40.01 ** | 19.04 ± 6.57 |
| AUMC(0–t) | h·h·mg/L | 160.74 ± 60.78 ** | 56.13 ± 18.01 |
| AUMC(0–∞) | h·h·mg/L | 189.92 ± 70.59 ** | 105.49 ± 31.13 |
| MRT(0–t) | h | 1.38 ± 0.41 ** | 3.53 ± 1.28 |
| MRT(0–∞) | h | 1.57 ± 0.51 ** | 5.54 ± 1.95 |
| t1/2z | h | 1.00 ± 0.40 ** | 4.02 ± 1.09 |
| Tmax | h | 0.03 ± 0.01 ** | 0.83 ± 0.24 |
| CLz/F | L/h/kg | 0.04 ± 0.01 ** | 2.63 ± 0.98 |
| Cmax | mg/L | 77.46 ± 23.82 ** | 5.75 ± 1.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Chen, X.; Zhao, G.; Tang, T.; Dong, W.; Wang, C.; Zhang, J.; Liao, Z. Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells. Pharmaceutics 2018, 10, 236. https://doi.org/10.3390/pharmaceutics10040236
Liang X, Chen X, Zhao G, Tang T, Dong W, Wang C, Zhang J, Liao Z. Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells. Pharmaceutics. 2018; 10(4):236. https://doi.org/10.3390/pharmaceutics10040236
Chicago/Turabian StyleLiang, Xinli, Xulong Chen, Guowei Zhao, Tao Tang, Wei Dong, Chunyan Wang, Jing Zhang, and Zhenggen Liao. 2018. "Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells" Pharmaceutics 10, no. 4: 236. https://doi.org/10.3390/pharmaceutics10040236
APA StyleLiang, X., Chen, X., Zhao, G., Tang, T., Dong, W., Wang, C., Zhang, J., & Liao, Z. (2018). Preparation, Characterization, and Pharmacokinetic Evaluation of Imperatorin Lipid Microspheres and Their Effect on the Proliferation of MDA-MB-231 Cells. Pharmaceutics, 10(4), 236. https://doi.org/10.3390/pharmaceutics10040236