Comparative Study on Excretive Characterization of Main Components in Herb Pair Notoginseng-Safflower and Single Herbs by LC–MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Excretion Experiments
2.4. Preparation of Calibration Standard and Quality Control (QC) Samples
2.5. Sample Preparation
2.6. LC–MS/MS Analysis
2.7. LC–MS/MS Method Validation
2.8. Data and Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.1.1. Specificity
3.1.2. Calibration Curve and Sensitivity
3.1.3. Precision and Accuracy
3.1.4. Matrix Effect and Extraction Recovery
3.1.5. Stability
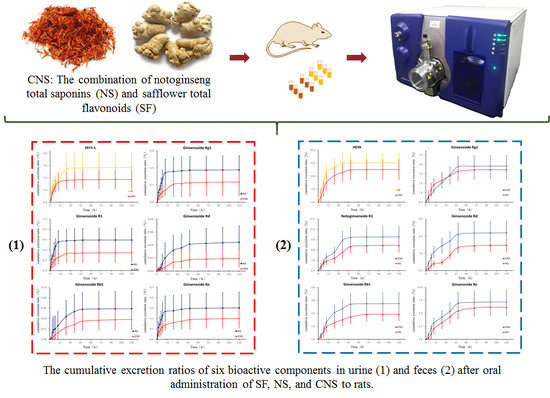
3.2. Excretion Study
3.2.1. Urinary Excretion Study
3.2.2. Fecal Excretion Study
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.J.; Zhu, J.Y.; Sun, M.Y.; Song, Y.N.; Rahman, K.; Peng, C.; Zhang, M.; Ye, Y.M.; Zhang, H. Anti-inflammatory effect of Man-Pen-Fang, a Chinese herbal compound, on chronic pelvic inflammation in rats. J. Ethnopharmacol. 2017, 208, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.P.; Lin, W.L.; Tsai, T.H. Pharmacokinetic interactions of herbal medicines for the treatment of chronic hepatitis. J. Food Drug Anal. 2017, 25, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, C.; Wu, C.; Du, K.; Zhang, J.; Qin, H.; Hou, J.; Du, G. HPLC-MS and HPLC-MS/MS analysis of seven active constituents of xiao-xu-ming decoction and application to a pharmacokinetic study after oral administration to rat. Acta Pharm. Sin. B 2012, 2, 186–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, D.; Huang, Y.; Li, L.; Mao, J.; Tian, J.; Ding, J. Pharmacokinetic evaluation of shenfu injection in beagle dogs after intravenous drip administration. Acta Pharm. Sin. B 2016, 6, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.L.; Fan, R. Effect of Pingchuan Guben decoction on patients with chronic obstructive pulmonary disease: Results from a randomized comparative effectiveness research trial. Exp. Ther. Med. 2017, 14, 3915–3925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, D.; Ganguli, A.; Dastidar, D.G.; Acharya, B.R.; Das, A.; Chakrabarti, G. Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin. Biochimie 2013, 95, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Guan, Y.M.; Lu, X.P.; Liang, X.L.; Chen, L.H. Mechanisms of P-Glycoprotein Modulation by Semen Strychni Combined with Radix Paeoniae Alba. Evid. Based Complement. Altern. Med. 2017, 2017, 1743870. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Li, H.X.; Ma, X.; Zhang, K.; Ma, Z.Z.; Jiang, Y.; Tu, P.F. Evaluation of the anti-myocardial ischemia effect of individual and combined extracts of Panax notoginseng and Carthamus tinctorius in rats. J. Ethnopharmacol. 2013, 145, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Du, Z.; Li, Y.; Wang, L.; Gao, P.; Gao, X.; Li, C.; Zhao, M.; Jiang, Y.; Tu, P.; et al. Integration of metabolomics with pharmacodynamics to elucidate the anti-myocardial ischemia effects of combination of notoginseng total saponins and safflower total flavonoids. Front. Pharmacol. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, D.P.; Qin, Z.; Wang, P.Y.; Hu, B.H.; Yu, J.G.; Zhao, Y.; Cai, B.; Chen, Y.L.; Lu, M.; et al. Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur. J. Pharmacol. 2018, 818, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Li, X.H.; Yang, Z.C. Effects of Panax notoginseng saponins on myocardial Gsalpha mRNA expression and ATPase activity after severe scald in rats. Burns 2003, 29, 541–546. [Google Scholar] [CrossRef]
- Yue, Q.X.; Xie, F.B.; Song, X.Y.; Wu, W.Y.; Jiang, B.H.; Guan, S.H.; Yang, M.; Liu, X.; Guo, D.A. Proteomic studies on protective effects of salvianolic acids, notoginsengnosides and combination of salvianolic acids and notoginsengnosides against cardiac ischemic-reperfusion injury. J. Ethnopharmacol. 2012, 141, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Liu, J.C.; Chen, Y.L.; Chen, C.H.; Lin, H.; Lin, J.W.; Chiu, W.T.; Chen, T.T.; Cheng, T.H. Inhibitory effect of trilinolein on endothelin-1-induced c-fos gene expression in cultured neonatal rat cardiomyocytes. Naunyn. Schmiedebergs. Arch. Pharmacol. 2005, 372, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Qiu, L.; Yu, Y.; Wang, C. Saponins of Panax notoginseng: Chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin. Investig. Drugs 2014, 23, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Yan, C.L.; Liu, H.X.; Wang, T.Y.; Shi, X.H.; Liu, J.P.; Orgah, J.; Fan, G.W.; Han, J.H.; Wang, X.Y.; et al. Network pharmacology exploration reveals endothelial inflammation as a common mechanism for stroke and coronary artery disease treatment of Danhong injection. Sci. Rep. 2017, 7, 15427. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Siu, W.S.; Fung, C.H.; Cheng, L.; Wong, C.W.; Zhang, C.; Liu, C.L.; Kwok, H.F.; Lau, C.P.; Wat, E.; et al. Pro-angiogenic effects of Carthami Flos whole extract in human microvascular endothelial cells in vitro and in zebrafish in vivo. Phytomedicine 2014, 21, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.C.; Lin, J.Y.; Lai, C.H.; Kuo, C.H.; Lin, W.Y.; Tsai, C.H.; Tsai, F.J.; Cheng, Y.C.; Peng, W.H.; Huang, C.Y. Carthamus tinctorius L. prevents LPS-induced TNFalpha signaling activation and cell apoptosis through JNK1/2-NFkappaB pathway inhibition in H9c2 cardiomyoblast cells. J. Ethnopharmacol. 2010, 130, 505–513. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Chinese Publishing Company of Chemical Industry: Beijing, China, 2015; p. 151. [Google Scholar]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Choi, J.S. The promotive effects of antioxidative apigenin on the bioavailability of paclitaxel for oral delivery in rats. Biomol. Ther (Seoul) 2010, 18, 469–476. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Liao, Z.G.; Zhu, J.Y.; Zhao, G.W.; Yang, M.; Yin, R.L.; Cao, Y.C.; Zhang, J.; Zhao, L.J. The absorption characterization effects and mechanism of Radix Angelicae dahuricae extracts on baicalin in Radix Scutellariae using in vivo and in vitro absorption models. J. Ethnopharmacol. 2012, 139, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Pu, Z.J.; Zhou, G.S.; Wang, J.; Zhu, Z.H.; Yue, S.J.; Li, J.P.; Shang, L.L.; Tang, Y.P.; Shi, X.Q.; et al. Comparative analysis of main bio-active components in the herb pair Danshen-Honghua and its single herbs by ultra-high performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry. J. Sep. Sci. 2017, 40, 3392–3401. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Du, F.; Gao, X.; Ma, X.; Huang, Y.; Xu, F.; Niu, W.; Wang, F.; Mao, Y.; et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Song, Y.L.; Guo, X.Y.; Tu, P.F.; Jiang, Y. Characterization of the herb-derived components in rats following oral administration of Carthamus tinctorius extract by extracting diagnostic fragment ions (DFIs) in the MS(n) chromatograms. Analyst 2014, 139, 6474–6485. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.A.; Xu, Z.S.; Ge, R.S. Effects of hydroxysafflor yellow A on the activity and mRNA expression of four CYP isozymes in rats. J. Ethnopharmacol. 2014, 151, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cheng, Y.; Li, T.; Dong, M.; Zhao, H.; Liu, G. Effects of notoginsenoside R1 on CYP1A2, CYP2C11, CYP2D1, and CYP3A1/2 activities in rats by cocktail probe drugs. Pharm Biol. 2016, 54, 231–236. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Q1 (Da) | Q3 (Da) | DP (V) | CE (eV) |
|---|---|---|---|---|
| HSYA | 611.1 | 491.0 | −150 | −36 |
| ginsenoside Rg1 | 845.6 | 799.5 | −85 | −38 |
| ginsenoside Rb1 | 1153.5 | 1107.4 | −103 | −37 |
| notoginsenoside R1 | 977.5 | 931.5 | −98 | −30 |
| ginsenoside Rd | 991.5 | 945.5 | −100 | −31 |
| ginsenoside Re | 991.5 | 945.5 | −130 | −37 |
| tenuifolin | 679.5 | 455.4 | −150 | −38 |
| Compounds | Matrix | Liner Range (ng/mL) | Regression Equation | Correlation Coefficient (r) | LLOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|---|---|---|---|
| HSYA | urine | 10–5000 | y = 0.0023x − 0.0011 | 0.9965 | 10.0 | 3.0 |
| feces | 2–5000 | y = 0.0093x + 0.007 | 0.9947 | 2.0 | 0.6 | |
| Ginsenoside Rg1 | urine | 10–5000 | y = 0.00092x + 0.00057 | 0.9976 | 10.0 | 3.0 |
| feces | 2–5000 | y = 0.0064x + 0.014 | 0.9950 | 2.0 | 0.6 | |
| Ginsenoside Rb1 | urine | 1–1000 | y = 0.00039x − 0.000059 | 0.9957 | 1.0 | 0.3 |
| feces | 2–5000 | y = 0.00035x + 0.00031 | 0.9909 | 2.0 | 0.6 | |
| Notoginsenoside R1 | urine | 1–1000 | y = 0.0012x + 0.000046 | 0.9950 | 1.0 | 0.3 |
| feces | 2–5000 | y = 0.009x + 0.021 | 0.9977 | 2.0 | 0.6 | |
| Ginsenoside Rd | urine | 1–1000 | y = 0.0047x + 0.00083 | 0.9903 | 1.0 | 0.3 |
| feces | 2–5000 | y = 0.0068x + 0.016 | 0.9971 | 2.0 | 0.6 | |
| Ginsenoside Re | urine | 1–1000 | y = 0.00087x + 0.00017 | 0.9969 | 1.0 | 0.3 |
| feces | 2–5000 | y = 0.0056x + 0.016 | 0.9970 | 2.0 | 0.6 |
| Compounds | QC conc. (ng/mL) | Intraday (n = 5) | Interday (n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Calc. conc (ng/mL) | Precision (%) | Accuracy (%) | Calc. conc (ng/mL) | Precision (%) | Accuracy (%) | ||
| HSYA | 10 | 10.2 | 6.7 | 1.7 | 10.2 | 9.3 | 2.4 |
| 200 | 207.6 | 3.1 | 3.7 | 215.7 | 3.9 | 7.9 | |
| 5000 | 5550.7 | 8.6 | 11.0 | 5566.0 | 7.6 | 11.4 | |
| Ginsenoside Rg1 | 10 | 9.7 | 7.4 | −2.8 | 10.0 | 10.2 | −0.7 |
| 200 | 227.1 | 2.3 | 3.5 | 229.8 | 2.6 | 14.9 | |
| 5000 | 5620.8 | 6.1 | 12.5 | 5634.0 | 6.0 | 12.9 | |
| Ginsenoside Rb1 | 1 | 1.0 | 8.3 | 1.2 | 1.0 | 14.0 | −8.5 |
| 20 | 19.9 | 8.1 | −0.6 | 21.2 | 6.4 | 5.4 | |
| 500 | 507.3 | 6.7 | 1.5 | 505.2 | 5.5 | 1.0 | |
| Notoginsenoide R1 | 1 | 1.0 | 6.9 | −10.3 | 1.2 | 14.5 | 7.2 |
| 20 | 20.0 | 6.6 | −0.6 | 20.8 | 4.9 | 4.3 | |
| 500 | 502.6 | 6.3 | 0.6 | 487.0 | 6.6 | −2.8 | |
| Ginsenoside Rd | 1 | 1.0 | 13.8 | −13.5 | 1.0 | 14.0 | −1.5 |
| 20 | 17.4 | 4.6 | −13.3 | 20.9 | 5.2 | 4.4 | |
| 500 | 497.6 | 7.1 | −0.6 | 449.0 | 8.4 | −10.3 | |
| Ginsenoside Re | 1 | 0.9 | 4.7 | −6.6 | 1.1 | 10.2 | 1.3 |
| 20 | 17.0 | 9.5 | −14.9 | 19.7 | 11.6 | −2.0 | |
| 500 | 475.4 | 8.7 | −4.9 | 471.1 | 6.2 | 9.8 | |
| Compounds | QC conc. (ng/mL) | Intraday (n = 5) | Interday (n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Calc. conc (ng/mL) | Precision (%) | Accuracy (%) | Calc. conc (ng/mL) | Precision (%) | Accuracy (%) | ||
| HSYA | 5 | 5.0 | 10.0 | 6.4 | 5.4 | 5.5 | 6.9 |
| 200 | 188.5 | 5.7 | −5.9 | 183.2 | 5.2 | −8.4 | |
| 2000 | 1756.7 | 2.8 | −9.2 | 1820.0 | 0.8 | −9.0 | |
| Ginsenoside Rg1 | 5 | 5.4 | 4.2 | 7.3 | 5.4 | 2.8 | 8.6 |
| 200 | 198.0 | 5.7 | −0.9 | 188.4 | 5.9 | −5.8 | |
| 2000 | 1773.3 | 3.0 | −9.6 | 1797.5 | 2.0 | −10.1 | |
| Ginsenoside Rb1 | 5 | 5.4 | 4.8 | 8.8 | 5.4 | 5.5 | 7.3 |
| 200 | 216.5 | 4.2 | 8.5 | 191.7 | 10.4 | −4.1 | |
| 2000 | 2101.7 | 4.6 | 5.3 | 1975.6 | 7.3 | −1.2 | |
| Notoginsenoside R1 | 5 | 5.4 | 5.5 | 7.5 | 5.3 | 5.7 | 6.3 |
| 200 | 201.2 | 4.2 | 0.6 | 196.4 | 6.6 | −1.8 | |
| 2000 | 1911.7 | 6.5 | −4.5 | 1813.3 | 5.8 | −9.3 | |
| Ginsenoside Rd | 5 | 5.2 | 8.0 | 3.0 | 5.2 | 8.1 | 4.0 |
| 200 | 198.0 | 4.30 | −1.0 | 191.1 | 7.3 | −4.4 | |
| 2000 | 1920.0 | 5.64 | −4.0 | 1810.0 | 5.8 | −9.5 | |
| Ginsenoside Re | 5 | 5.1 | 7.8 | 1.1 | 5.1 | 6.5 | 2.2 |
| 200 | 199.7 | 5.8 | −0.3 | 195.5 | 7.3 | −2.2 | |
| 2000 | 1965.0 | 5.4 | −1.6 | 1882.8 | 5.1 | −5.9 | |
| Compounds | Urine | Feces | ||||
|---|---|---|---|---|---|---|
| QC conc. (ng/mL) | Matrix Effect (%) | Recovery (%) | QC conc. (ng/mL) | Matrix Effect (%) | Recovery (%) | |
| HSYA | 10 | 89.9 | 90.8 | 5 | 99.1 | 92.8 |
| 200 | 90.4 | 91.8 | 200 | 95.7 | 91.5 | |
| 5000 | 103.7 | 101.6 | 2000 | 99.9 | 97.5 | |
| Ginsenoside Rg1 | 10 | 97.7 | 96.4 | 5 | 88.5 | 84.6 |
| 200 | 101.6 | 101.4 | 200 | 94.3 | 90.9 | |
| 5000 | 93.1 | 90.2 | 2000 | 91.3 | 90.5 | |
| Ginsenoside Rb1 | 1 | 104.4 | 92.5 | 5 | 94.1 | 88.9 |
| 20 | 109.3 | 98.2 | 200 | 96.3 | 91.9 | |
| 500 | 93.3 | 92.0 | 2000 | 90.7 | 89.0 | |
| Notoginsenoside R1 | 1 | 90.6 | 96.3 | 5 | 93.3 | 90.6 |
| 20 | 90.9 | 90.7 | 200 | 103.1 | 101.9 | |
| 500 | 94.2 | 91.8 | 2000 | 91.0 | 88.3 | |
| Ginsenoside Rd | 1 | 96.8 | 94.7 | 5 | 91.8 | 87.4 |
| 20 | 105.9 | 96.0 | 200 | 91.9 | 90.3 | |
| 500 | 108.0 | 104.6 | 2000 | 90.8 | 89.6 | |
| Ginsenoside Re | 1 | 98.8 | 93.2 | 5 | 91.2 | 89.6 |
| 20 | 90.5 | 91.0 | 200 | 104.1 | 102.5 | |
| 500 | 103.4 | 100.2 | 2000 | 92.0 | 90.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-Y.; Song, J.-Y.; Li, Y.; Meng, Y.-Q.; Zhao, M.-B.; Jiang, Y.; Tu, P.-F.; Guo, X.-Y. Comparative Study on Excretive Characterization of Main Components in Herb Pair Notoginseng-Safflower and Single Herbs by LC–MS/MS. Pharmaceutics 2018, 10, 241. https://doi.org/10.3390/pharmaceutics10040241
Lu Y-Y, Song J-Y, Li Y, Meng Y-Q, Zhao M-B, Jiang Y, Tu P-F, Guo X-Y. Comparative Study on Excretive Characterization of Main Components in Herb Pair Notoginseng-Safflower and Single Herbs by LC–MS/MS. Pharmaceutics. 2018; 10(4):241. https://doi.org/10.3390/pharmaceutics10040241
Chicago/Turabian StyleLu, Ying-Yuan, Jin-Yang Song, Yan Li, Yu-Qing Meng, Ming-Bo Zhao, Yong Jiang, Peng-Fei Tu, and Xiao-Yu Guo. 2018. "Comparative Study on Excretive Characterization of Main Components in Herb Pair Notoginseng-Safflower and Single Herbs by LC–MS/MS" Pharmaceutics 10, no. 4: 241. https://doi.org/10.3390/pharmaceutics10040241
APA StyleLu, Y. -Y., Song, J. -Y., Li, Y., Meng, Y. -Q., Zhao, M. -B., Jiang, Y., Tu, P. -F., & Guo, X. -Y. (2018). Comparative Study on Excretive Characterization of Main Components in Herb Pair Notoginseng-Safflower and Single Herbs by LC–MS/MS. Pharmaceutics, 10(4), 241. https://doi.org/10.3390/pharmaceutics10040241