Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials
Abstract
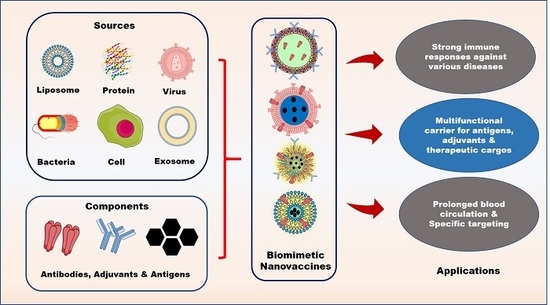
:1. Introduction
2. Components of Biomimetic Immunomodulatory Nanovaccines
2.1. Types of Biomimetic Nanoparticles (Nps)
2.1.1. Liposomes
2.1.2. Virus-Like Particles (VLPs)
2.1.3. Self-assembling Protein Nanoparticles (NPs)
2.1.4. Cell Membrane-Decorated Nanoparticles (NPs)
2.1.5. Exosomes
2.2. Cargoes Used for Immunomodulatory Nanovaccines
2.2.1. Adjuvants
2.2.2. Detained Bacterial Toxins
3. Advantages of Nanovaccines
4. Applications of Biomimetic Nanovaccines
4.1. Anti-Bacterial Therapy
4.2. Anti-HIV Therapy
4.3. Anti-Malarial Therapy
4.4. Anti-Tumor Immunity
4.5. Anti-Melittin Therapy
4.6. Foot-and-Mouth Disease Virus Therapy
5. Challenges and Future Directions of Biomimetic Nanovaccines
6. Clinical Aspects of Biomimetic Nanovaccines
7. Conclusions
Funding
Conflicts of Interest
References
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Larché, M.; Wraith, D.C. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005, 11, S69. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol. Lett. 2009, 122, 134–136. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.P.; Jabri, B. Vaccine against autoimmune disease: Antigen-specific immunotherapy. Curr. Opin. Immunol. 2013, 25, 410–417. [Google Scholar] [CrossRef]
- Anderton, S.M.; Wraith, D.C. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur. J. Immunol. 1998, 28, 1251–1261. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [Green Version]
- Whitney, C.G.; Zhou, F.; Singleton, J.; Schuchat, A. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 352. [Google Scholar]
- Lee, H.B.; Yoon, S.Y.; Singh, B.; Oh, S.H.; Cui, L.; Yan, C.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Oral Immunization of FMDV Vaccine Using pH-Sensitive and Mucoadhesive Thiolated Cellulose Acetate Phthalate Microparticles. Tissue Eng. Regen. Med. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Glenny, A.T.; Pope, C.G.; Waddington, H.; Wallace, U. Immunological notes. XVII–XXIV. J. Pathol. Bacteriol. 1926, 29, 31–40. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Opie, E.L.; Freund, J. An Experimental Study of Protective Inoculation with Heat Killed Tubercle Bacilli. J. Exp. Med. 1937, 66, 761. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, Y.; Geary, S.M.; Lemke, C.D.; Salem, A.K. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011, 28, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Dahle, C.E.; Baman, N.K.; Rich, N.; Weiner, G.J.; Salem, A.K. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J. Immunother. 2007, 30, 469–478. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly(ε-caprolactone)-Poly(ethylene glycol)-Poly(ε-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 735–750. [Google Scholar] [CrossRef]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine delivery systems: Size matters. AAPS J. 2013, 15, 85–94. [Google Scholar] [CrossRef]
- Bishop, C.J.; Kozielski, K.L.; Green, J.J. Exploring the role of polymer structure on intracellular nucleic acid delivery via polymeric nanoparticles. J. Control. Release 2015, 219, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Fang, R.H.; Luk, B.T.; Hu, C.M.J.; Thamphiwatana, S.; Dehaini, D.; Angsantikul, P.; Kroll, A.V.; Pang, Z.; Gao, W. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater. 2016, 26, 1628–1635. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Polhemus, M.E.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Antigen-Displaying Lipid-Enveloped PLGA Nanoparticles as Delivery Agents for a Plasmodium vivax Malaria Vaccine. PLoS ONE 2012, 7, e31472. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.K.; Pandey, R.S. Immunological evaluation of colonic delivered Hepatitis B surface antigen loaded TLR-4 agonist modified solid fat nanoparticles. Int. Immunopharmacol. 2016, 39, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rizzo, M.; Bhattacharya, S.; Huang, L. Characterization of cationic lipid-protamine–DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998, 5, 930. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013, 8, 933–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberg, A.L.; Kennedy, R.B.; Li, P.; Ovsyannikova, I.G.; Poland, G.A. Systems biology approaches to new vaccine development. Curr. Opin. Immunol. 2011, 23, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Uthaman, S.; Huh, K.M.; Koh, Y.S.; Lee, S.; Park, I.K. Multimodal Composite Iron Oxide Nanoparticles for Biomedical Applications. Tissue Eng. Regen. Med. 2019, 16, 451–465. [Google Scholar] [CrossRef]
- Singh, R.K.; Kim, H.W. Inorganic nanobiomaterial drug carriers for medicine. Tissue Eng. Regen. Med. 2013, 10, 296–309. [Google Scholar] [CrossRef]
- Yahyapour, R.; Farhood, B.; Graily, G.; Rezaeyan, A.; Rezapoor, S.; Abdollahi, H.; Cheki, M.; Amini, P.; Fallah, H.; Najafi, M.; et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng. Regen. Med. 2018, 15, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Park, G.K.; McDonald, E.J.; Choi, H.S. Targeted Near-Infrared Fluorescence Imaging for Regenerative Medicine. Tissue Eng. Regen. Med. 2019, 16, 433–442. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2015, 11, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Su, J.; Zhang, L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv. Mater. 2013, 25, 3549–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M. Stem cells for therapy. Tissue Eng. Regen. Med. 2013, 10, 223–229. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.K. Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics. Polymers 2018, 10, 983. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Fang, R.H.; Zhang, L. Erythrocyte-Inspired Delivery Systems. Adv. Healthc. Mater. 2012, 1, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ran, D.; Campeau, A.; Xiao, C.; Zhou, J.; Dehaini, D.; Jiang, Y.; Kroll, A.V.; Zhang, Q.; Gao, W.; et al. Multiantigenic Nanotoxoids for Antivirulence Vaccination against Antibiotic-Resistant Gram-Negative Bacteria. Nano Lett. 2019, 19, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Frueh, J.; Wu, Z.; He, Q. How Leucocyte Cell Membrane Modified Janus Microcapsules are Phagocytosed by Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Guo, C.; Wang, J.; Korzun, W.J.; Wang, X.Y.; Ghosh, S.; Yang, H. Leutusome: A Biomimetic Nanoplatform Integrating Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing. Nano Lett. 2018, 18, 6164–6174. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, G.; Ran, D.; Krishnan, N.; Fang, R.H.; Gao, W.; Spector, S.A.; Zhang, L. T-Cell-Mimicking Nanoparticles Can Neutralize HIV Infectivity. Adv. Mater. 2018, 30, 1802233. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, T.D.T.; Marasini, R.; Eliyapura, A.; Azizi, T.; Jaberi-Douraki, M.; Aryal, S. Biomimetic Natural Killer Membrane Camouflaged Polymeric Nanoparticle for Targeted Bioimaging. Adv. Funct. Mater. 2019, 29, 1806817. [Google Scholar] [CrossRef]
- Hu, C.M.J.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Dai, L.; He, Q.; Li, J. Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv. Healthc. Mater. 2015, 4, 1645–1652. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zheng, D.W.; Zhang, M.K.; Yu, W.Y.; Qiu, W.X.; Hu, J.J.; Feng, J.; Zhang, X.Z. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016, 16, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Wang, Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 137–149. [Google Scholar] [CrossRef]
- Kang, S.; Pushko, P.; Bright, R.; Smith, G.; Compans, R. Influenza virus-like particles as pandemic vaccines. In Vaccines for Pandemic Influenza; Springer: Berlin, Germany, 2009; pp. 269–289. [Google Scholar]
- Castón, J.R.; Carrascosa, J.L. The basic architecture of viruses. In Structure and Physics of Viruses; Springer: Berlin, Germany, 2013; pp. 53–75. [Google Scholar]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, S.J.; Sitaraman, K.; Young, H.A.; Hughes, S.H.; Chatterjee, D.K. Protein delivery using engineered virus-like particles. Proc. Natl. Acad. Sci. USA 2011, 108, 16998. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yeh, Y.C.; Chan, J.T.; Yang, Y.C.; Yang, J.R.; Liu, M.T.; Wu, H.S.; Hsiao, P.W. A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus. PLoS ONE 2012, 7, e42363. [Google Scholar] [CrossRef]
- Bai, H.; Wang, C.; Qi, Y.; Xu, J.; Li, N.; Chen, L.; Jiang, B.; Zhu, X.; Zhang, H.; Li, X.; et al. Major vault protein suppresses lung cancer cell proliferation by inhibiting STAT3 signaling pathway. BMC Cancer 2019, 19, 454. [Google Scholar] [CrossRef]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.J.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Korsholm, K.S.; Andersen, P.; Perrie, Y.; Christensen, D. Liposomal vaccine delivery systems. Expert Opin. Drug Deliv. 2011, 8, 505–519. [Google Scholar] [CrossRef]
- Heurtault, B.; Frisch, B.; Pons, F. Liposomes as delivery systems for nasal vaccination: Strategies and outcomes. Expert Opin. Drug Deliv. 2010, 7, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Lee, S.H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Mukkur, T.; Benson, H.A.; Chen, Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J. Pharm. Sci. 2009, 98, 812–843. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Goyal, A.K.; Gupta, P.N.; Mishra, N.; Mehta, A.; Vyas, S.P. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine 2008, 26, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Amacker, M.; Zurbriggen, R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev. Vaccines 2011, 10, 437–446. [Google Scholar] [CrossRef]
- Li Sd Huang, L.Y. In vivo gene transfer via intravenous administration of cationic lipid–protamine–DNA (LPD) complexes. Gene Ther. 1997, 4, 891. [Google Scholar]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhou, J.; Chen, S.; Cai, L.L.; Bao, Q.Y.; Zheng, F.Y.; Lu, J.Q.; Padmanabha, J.; Hengst, K.; Malcolm, K.; et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine 2000, 18, 1051–1058. [Google Scholar] [CrossRef]
- André, F.E. Overview of a 5-year clinical experience with a yeast-derived hepatitis B vaccine. Vaccine 1990, 8, S74–S78. [Google Scholar] [CrossRef]
- Cutts, F.T.; Franceschi, S.; Goldie, S.; Castellsague, X.; de Sanjose, S.; Garnett, G.; Edmunds, W.J.; Claeys, P.; Goldenthal, K.L.; Harper, D.M.; et al. Human papillomavirus and HPV vaccines: A review. Bull. World Health Organ. 2007, 85, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Kingsman, S.M.; Kingsman, A.J. Polyvalent recombinant antigens: A new vaccine strategy. Vaccine 1988, 6, 304–306. [Google Scholar] [CrossRef]
- Maurer, P.; Jennings, G.T.; Willers, J.; Rohner, F.; Lindman, Y.; Roubicek, K.; Renner, W.A.; Müller, P.; Bachmann, M.F. A therapeutic vaccine for nicotine dependence: Preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunol. 2005, 35, 2031–2040. [Google Scholar] [CrossRef]
- Hu, Y.C. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol. Sin. 2005, 26, 405–416. [Google Scholar] [CrossRef]
- Andrews, S.C. The Ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 691–705. [Google Scholar] [CrossRef]
- Chasteen, N.D.; Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102. [Google Scholar] [CrossRef]
- Champion, C.I.; Kickhoefer, V.A.; Liu, G.; Moniz, R.J.; Freed, A.S.; Bergmann, L.L.; Vaccari, D.; Raval-Fernandes, S.; Chan, A.M.; Rome, L.H. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS ONE 2009, 4, e5409. [Google Scholar] [CrossRef]
- Wahome, N.; Pfeiffer, T.; Ambiel, I.; Yang, Y.; Keppler, O.T.; Bosch, V.; Burkhard, P. Conformation-specific Display of 4E10 and 2F5 Epitopes on Self-assembling Protein Nanoparticles as a Potential HIV Vaccine. Chem. Biol. Drug Des. 2012, 80, 349–357. [Google Scholar] [CrossRef]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, H.S. Exosomes as Therapeutic Vehicles for Cancer. Tissue Eng. Regen. Med. 2019, 16, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, A.L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, X.; Bao, J.; Wang, Y.; Liu, H.; Tang, L. Exosomes in pathogen infections: A bridge to deliver molecules and link functions. Front. Immunol. 2018, 9, 90. [Google Scholar] [CrossRef]
- Cox, J.C.; Coulter, A.R. Adjuvants—A classification and review of their modes of action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef]
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef]
- Fraser, C.K.; Diener, K.R.; Brown, M.P.; Hayball, J.D. Improving vaccines by incorporating immunological coadjuvants. Expert Rev. Vaccines 2007, 6, 559–578. [Google Scholar] [CrossRef]
- Riitho, V.; Walters, A.A.; Somavarapu, S.; Lamp, B.; Rümenapf, T.; Krey, T.; Rey, F.A.; Oviedo-Orta, E.; Stewart, G.R.; Locker, N.; et al. Design and evaluation of the immunogenicity and efficacy of a biomimetic particulate formulation of viral antigens. Sci. Rep. 2017, 7, 13743. [Google Scholar] [CrossRef]
- Le, Q.V.; Suh, J.; Choi, J.J.; Park, G.T.; Lee, J.W.; Shim, G.; Oh, Y.K. In Situ Nanoadjuvant-Assembled Tumor Vaccine for Preventing Long-Term Recurrence. ACS Nano 2019, 13, 7442–7462. [Google Scholar] [CrossRef]
- Siefert, A.L.; Caplan, M.J.; Fahmy, T.M. Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials 2016, 97, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.W.; Huang, C.Y.; Yao, B.Y.; Lin, J.C.; Agrawal, A.; Algaissi, A.; Peng, B.H.; Liu, Y.H.; Huang, P.H.; Juang, R.H. Viromimetic STING Agonist-Loaded Hollow Polymeric Nanoparticles for Safe and Effective Vaccination against Middle East Respiratory Syndrome Coronavirus. Adv. Funct. Mater. 2019, 29, 1807616. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Sun, Y.; Zhou, P.; Wang, Y.; Zhang, Y. Dendritic cell targeted vaccines: Recent progresses and challenges. Hum. Vaccines Immunother. 2016, 12, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mohan, T.; Chang, T.Z.; Gonzalez, G.X.; Wang, Y.; Kwon, Y.M.; Kang, S.M.; Compans, R.W.; Champion, J.A.; Wang, B.Z. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 2018, 9, 359. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787. [Google Scholar] [CrossRef]
- Yu, W.; He, X.; Yang, Z.; Yang, X.; Xiao, W.; Liu, R.; Xie, R.; Qin, L.; Gao, H. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials 2019, 217, 119309. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef]
- Dhanasooraj, D.; Kumar, R.A.; Mundayoor, S. Vaccine delivery system for tuberculosis based on nano-sized hepatitis B virus core protein particles. Int. J. Nanomed. 2013, 8, 835. [Google Scholar]
- Bai, J.; Yang, E.; Chang, P.S.; Ryu, S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzym. Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Esté, J.A.; Cihlar, T. Current status and challenges of antiretroviral research and therapy. Antivir. Res. 2010, 85, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mamo, T.; Moseman, E.A.; Kolishetti, N.; Salvador-Morales, C.; Shi, J.; Kuritzkes, D.R.; Langer, R.; Andrian Uv Farokhzad, O.C. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine 2010, 5, 269–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, D.; Boxi, A.; Ashe, S.; Thathapudi, N.C.; Nayak, B. Stavudine loaded gelatin liposomes for HIV therapy: Preparation, characterization and in vitro cytotoxic evaluation. Mater. Sci. Eng. C 2017, 73, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Dykers, T.; Hu, S.L.; Faltynek, C.R.; Ho, R. Effect of MTP-PE liposomes and interleukin-7 on induction of antibody and cell-mediated immune responses to a recombinant HIV-envelope protein. J. Acquir. Immune Defic. Syndr. 1994, 7, 799–806. [Google Scholar] [PubMed]
- Hanson, M.C.; Abraham, W.; Crespo, M.P.; Chen, S.H.; Liu, H.; Szeto, G.L.; Kim, M.; Reinherz, E.L.; Irvine, D.J. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine 2015, 33, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Poteet, E.; Lewis, P.; Chen, C.; Ho, S.O.; Do, T.; Chiang, S.; Labranche, C.; Montefiori, D.; Fujii, G.; Yao, Q. Toll-like receptor 3 adjuvant in combination with virus-like particles elicit a humoral response against HIV. Vaccine 2016, 34, 5886–5894. [Google Scholar] [CrossRef]
- Andersson, A.M.C.; Ragonnaud, E.; Seaton, K.E.; Sawant, S.; Folgori, A.; Colloca, S.; Labranche, C.; Montefiori, D.C.; Tomaras, G.D.; Holst, P.J. Effect of HIV-1 envelope cytoplasmic tail on adenovirus primed virus encoded virus-like particle immunizations. Vaccine 2016, 34, 5344–5351. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, J.M.; Nkolola, J.P.; Peng, H.; Cheung, A.; Perry, J.; Miller, C.A.; Seaman, M.S.; Barouch, D.H.; Chen, B. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. USA 2012, 109, 12111–12116. [Google Scholar] [CrossRef] [Green Version]
- Pejawar-Gaddy, S.; Kovacs, J.M.; Barouch, D.H.; Chen, B.; Irvine, D.J. Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in HIV vaccination. Bioconjugate Chem. 2014, 25, 1470–1478. [Google Scholar] [CrossRef]
- Campbell, E.M.; Hope, T.J. HIV-1 capsid: The multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.; Peoples, V.; Johnson, F.; Bibbs, R.; Topps, D.; Bopda-Waffo, A.; Coats, M. Utilizing nanotechnology to combat malaria. J. Infect. Dis. Ther. 2015, 3, 229. [Google Scholar]
- Rahman, K.; Khan, S.U.; Fahad, S.; Chang, M.X.; Abbas, A.; Khan, W.U.; Rahman, L.; Haq, Z.U.; Nabi, G.; Khan, D. Nano-biotechnology: A new approach to treat and prevent malaria. Int. J. Nanomed. 2019, 14, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Tagami, T.; Ozeki, T. Effective-Loading of Platinum–Chloroquine into PEGylated Neutral and Cationic Liposomes as a Drug Delivery System for Resistant Malaria Parasites. Biol. Pharm. Bull. 2017, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.; Huiskamp, C.W.; Eling, W.; Crommelin, D. Chloroquine containing liposomes in the chemotherapy of murine malaria. Parasitology 1989, 98, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Urbán, P.; Estelrich, J.; Cortés, A.; Fernàndez-Busquets, X. A nanovector with complete discrimination for targeted delivery to Plasmodium falciparum-infected versus non-infected red blood cells in vitro. J. Control. Release 2011, 151, 202–211. [Google Scholar] [CrossRef]
- Marques, J.; Valle-Delgado, J.J.; Urbán, P.; Baró, E.; Prohens, R.; Mayor, A.; Cisteró, P.; Delves, M.; Sinden, R.E.; Grandfils, C. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, R.K.; Garg, N.K.; Dalai, S.K.; Awasthi, A. Transdermal immunization of P. falciparum surface antigen (MSP-119) via elastic liposomes confers robust immunogenicity. Hum. Vaccines Immunother. 2016, 12, 990–992. [Google Scholar] [CrossRef]
- Seth, L.; Ferlez, K.M.B.; Kaba, S.A.; Musser, D.M.; Emadi, S.; Matyas, G.R.; Beck, Z.; Alving, C.R.; Burkhard, P.; Lanar, D.E. Development of a self-assembling protein nanoparticle vaccine targeting Plasmodium falciparum Circumsporozoite Protein delivered in three Army Liposome Formulation adjuvants. Vaccine 2017, 35, 5448–5454. [Google Scholar] [CrossRef]
- Leach, A.; Vekemans, J.; Lievens, M.; Ofori-Anyinam, O.; Cahill, C.; Owusu-Agyei, S.; Abdulla, S.; Macete, E.; Njuguna, P.; Savarese, B. Design of a phase III multicenter trial to evaluate the efficacy of the RTS, S/AS01 malaria vaccine in children across diverse transmission settings in Africa. Malar. J. 2011, 10, 224. [Google Scholar] [CrossRef]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, S.; Mohapatra, A.; Singh, B.; Revuri, V.; Lee, Y.K.; Kim, C.; Cho, C.S.; Park, I.K. Self-assembled, adjuvant/antigen-based nanovaccine mediates anti-tumor immune response against melanoma tumor. Polymers 2018, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.V.; Jiang, Y.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanoparticle Vaccines for Cancer Therapy. Adv. Biosyst. 2019, 3, 1800219. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, Y.Y.; Kang, W.J.; Kim, H.C.; Ku, S.Y.; Kang, B.C.; Yun, J.W. The Use of Normal Stem Cells and Cancer Stem Cells for Potential Anti-Cancer Therapeutic Strategy. Tissue Eng. Regen. Med. 2018, 15, 365–380. [Google Scholar] [CrossRef]
- Lou, J.; Zhang, L.; Zheng, G. Advancing Cancer Immunotherapies with Nanotechnology. Adv. Ther. 2019, 2, 1800128. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef]
- Ichihashi, T.; Satoh, T.; Sugimoto, C.; Kajino, K. Emulsified phosphatidylserine, simple and effective peptide carrier for induction of potent epitope-specific T cell responses. PLoS ONE 2013, 8, e60068. [Google Scholar] [CrossRef]
- Talesh, G.A.; Ebrahimi, Z.; Badiee, A.; Mansourian, M.; Attar, H.; Arabi, L.; Jalali, S.A.; Jaafari, M.R. Poly (I: C)-DOTAP cationic nanoliposome containing multi-epitope HER2-derived peptide promotes vaccine-elicited anti-tumor immunity in a murine model. Immunol. Lett. 2016, 176, 57–64. [Google Scholar] [CrossRef]
- Shariat, S.; Badiee, A.; Jalali, S.A.; Mansourian, M.; Yazdani, M.; Mortazavi, S.A.; Jaafari, M.R. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014, 355, 54–60. [Google Scholar] [CrossRef]
- Chen, M.; Chen, M.; He, J. Cancer cell membrane cloaking nanoparticles for targeted co-delivery of doxorubicin and PD-L1 siRNA. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1635–1641. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.; Wang, C.; Zhang, X. Erythrocyte–Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.V.; Fang, R.H.; Jiang, Y.; Zhou, J.; Wei, X.; Yu, C.L.; Gao, J.; Luk, B.T.; Dehaini, D.; Gao, W. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater. 2017, 29, 1703969. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jiang, Q.; Xiao, X.; Xu, X.; Xu, Y.; Kong, Y.; Zhang, W.; Zeng, Y.; Liu, X.; Luo, B. Assisting anti-PD-1 antibody treatment with a liposomal system capable of recruiting immune cells. Nanoscale 2019, 11, 7996–8011. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2015, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Chen, J.Y.; Chen, H.W.; Hu, C.M.J. Nanoparticle Vaccines Adopting Virus-like Features for Enhanced Immune Potentiation. Nanotheranostics 2017, 1, 244–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.M.; Vartabedian, V.F.; Kim, M.C.; He, S.; Kang, S.M.; Selvaraj, P. Influenza virus-like particles engineered by protein transfer with tumor-associated antigens induces protective antitumor immunity. Biotechnol. Bioeng. 2015, 112, 1102–1110. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fiering, S.N.; Steinmetz, N.F. Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Adv. Ther. 2019, 2, 1900003. [Google Scholar] [CrossRef]
- Kang, T.; Li, C.; Du, T.; Wu, Y.; Yang, Y.; Liu, X.; Zhang, Q.; Xu, X.; Gou, M. A biomimetic nanoparticle-enabled toxoid vaccine against melittin. Int. J. Nanomed. 2018, 13, 3251–3261. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-Spectrum Neutralization of Pore-Forming Toxins with Human Erythrocyte Membrane-Coated Nanosponges. Adv. Healthc. Mater. 2018, 7, 1701366. [Google Scholar] [CrossRef]
- Dykman, L.A.; Staroverov, S.A.; Mezhenny, P.V.; Fomin, A.S.; Kozlov, S.V.; Volkov, A.A.; Laskavy, V.N.; Shchyogolev, S.Y. Use of a synthetic foot-and-mouth disease virus peptide conjugated to gold nanoparticles for enhancing immunological response. Gold Bull. 2015, 48, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.; Sun, S.; Chen, H.; Huang, J.; Du, P.; Dong, H.; Xu, X.; Mu, S.; Zhang, Z.; Guo, H. Golden-star nanoparticles as adjuvant effectively promotes immune response to foot-and-mouth disease virus-like particles vaccine. Vaccine 2018, 36, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhuang, Y.; Zhang, Y.; Luo, Z.; Gao, N.; Li, P.; Pan, H.; Cai, L.; Ma, Y. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3–IRF3 signaling and type I interferons in dendritic cells. Vaccine 2012, 30, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Rueda, F.; Simón, L.; Cordobilla, B.; Albericio, F.; Domingo, J.C. Liposomes containing NY-ESO-1/tetanus toxoid and adjuvant peptides targeted to human dendritic cells via the Fc receptor for cancer vaccines. Nanomedicine 2014, 9, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lila, A.S.A.; Kawaguchi, Y.; Shimazaki, Y.; Watanabe, Y.; Mima, Y.; Hashimoto, Y.; Okuhira, K.; Storm, G.; Ishima, Y. A Novel Platform for Cancer Vaccines: Antigen-Selective Delivery to Splenic Marginal Zone B Cells via Repeated Injections of PEGylated Liposomes. J. Immunol. 2018, 201, 2969–2976. [Google Scholar] [CrossRef]
- Fantappie, L.; de Santis, M.; Chiarot, E.; Carboni, F.; Bensi, G.; Jousson, O.; Margarit, I.; Grandi, G. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles 2014, 3, 24015. [Google Scholar] [CrossRef]
- Li, L.L.; Xu, J.H.; Qi, G.B.; Zhao, X.; Yu, F.; Wang, H. Core–shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and limitations of current techniques for analyzing the biodistribution of nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01, helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Smith, L.R.; Wloch, M.K.; Ye, M.; Reyes, L.R.; Boutsaboualoy, S.; Dunne, C.E.; Chaplin, J.A.; Rusalov, D.; Rolland, A.P.; Fisher, C.L. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010, 28, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Glück, R.; Metcalfe, I.C. New technology platforms in the development of vaccines for the future. Vaccine 2002, 20, B10–B16. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Zitvogel, L.; Galluzzi, L. Victories and deceptions in tumor immunology. OncoImmunology 2013, 2, e23687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, P.A.; Shepherd, F.A. Immunotherapy for Lung Cancer. J. Thorac. Oncol. 2008, 3, S164–S170. [Google Scholar] [CrossRef] [Green Version]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]









| Nanoparticles | Components | Application | References |
|---|---|---|---|
| Liposomes | Liposome-polycation-DNA NPs | DNA vaccine delivery | [24] |
| PLGA NPs with lipid antigens | Malarial vaccine delivery | [21] | |
| Cancer cell membranes with lipids coated onto polymeric NPs | TLR 7 delivery: Anticancer vaccine | [52] | |
| VLPs | Avian retrovirus with Gag fusion proteins | Intracellular protein delivery | [57] |
| Genetically modified VLP | Anti-viral protection | [58] | |
| Self-assembling proteins | Hollow vault protein | Suppress lung cancer proliferation | [59] |
| Cell membrane decorated NPs | Gastric epithelial cell membrane coated PLGA NPs loaded with antibiotics | Anti-bacerial therapy | [60] |
| Bacterial membrane coated Gold NPs | Antibacterial immunity | [61] |
| Immune Potentiators | Delivery Systems |
|---|---|
| dsRNA: Poly (I:C), Poly-IC:LC MPLA (monophosphoryl lipid A) LPS (Lipopolysaccharide) CpG oligodeoxynucleotides Flagellin Imiquimod (R837) Resiquimod (848) Saponins (QS-21) | Aluminum salts Incomplete Freund’s reagents Virus-like particles Polylactic acid, Poly(lactic-co-glycolide) data |
| Type of Biomimetic Nanoparticle (NP) | Therapeutic Cargo | Application | Reference |
|---|---|---|---|
| Liposome | Hepa 1-6 cell lysate and Poly I:C | High tumor specific CTL immune response | [145] |
| P5 peptide and Poly I:C | CTL immune response and anti-cancer therapy | [130] | |
| Tumor associated ESO-1 antigen and IL-1, MAP-IFN-γ | Fcγ receptor targeting and anti-cancer therapy | [146] | |
| OVA antigen | CTL response and cancer immune therapy | [147] | |
| Endolysin | Degradation of bacterial protein and anti-bacterial therapy | [102] | |
| Env-2-3-SF2, IL-7 | Strong antibody response and anti-HIV therapy | [107] | |
| MPER and MPLA, STING, cdGMP | Strong T-cell response and anti-HIV therapy | [108] | |
| MSP-1 | Activation of epidermal APC | [121] | |
| Virus like NP | CFP 10 | CTL activity, Th1 immune response, and anti-bacterial therapy | [101] |
| HIV env antigen | Maintaining the germinal center, and releasing neutralizing antibody for anti-HIV therapy | [110] | |
| CSP-hepatitis B surface antigen and Abisco-100, Matrix-M | Targeting infected erythrocytes and CD8 + T-cell responses in anti-malaria therapy | [123] | |
| HER-2 antigen | Th1 & Th2 type antibody response and anti-cancer therapy | [139] | |
| Outer membrane coated nanovaccine | Alum adjuvant | Th2 type immune response anti-bacterial therapy | [148] |
| RBC membrane coated Nanovaccine | None | Adsorption of bacterial endotoxin | [149] |
| None | Natural substrate for PFT for Anti-melittin therapy | [142] | |
| T-cell coated Nanovaccine | None | Inhibition of viral attack to host cell | [113] |
| Cancer cell membrane coated Nanovaccine | PD-L 1 siRNA | Tumor targeting and anti-cancer therapy | [132] |
| CpG oligodeoxynucleotide | Stimulation of APC maturation and release of pro-inflammatory cytokines in anti-cancer therapy | [134] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayan, V.; Mohapatra, A.; Uthaman, S.; Park, I.-K. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics 2019, 11, 534. https://doi.org/10.3390/pharmaceutics11100534
Vijayan V, Mohapatra A, Uthaman S, Park I-K. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics. 2019; 11(10):534. https://doi.org/10.3390/pharmaceutics11100534
Chicago/Turabian StyleVijayan, Veena, Adityanarayan Mohapatra, Saji Uthaman, and In-Kyu Park. 2019. "Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials" Pharmaceutics 11, no. 10: 534. https://doi.org/10.3390/pharmaceutics11100534
APA StyleVijayan, V., Mohapatra, A., Uthaman, S., & Park, I. -K. (2019). Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics, 11(10), 534. https://doi.org/10.3390/pharmaceutics11100534