Preparation, Characterisation, and Topical Delivery of Terbinafine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Conversion of TBF Hydrochloride to TBF-Free Base
2.2.2. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.2.3. HPLC Analysis and Method Validation
2.2.4. Solubility Parameters, Solubility, and Stability Studies
2.2.5. Dynamic Vapour Sorption Studies
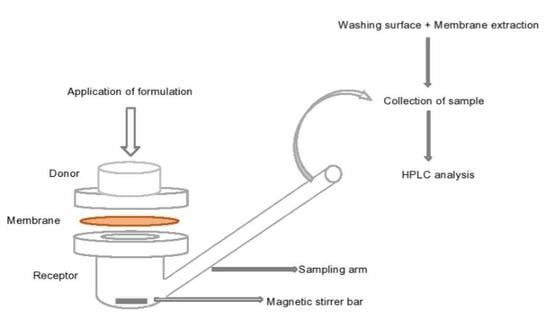
2.2.6. Permeation and Mass Balance Studies of TBF-Free Base (1% w/w) in Porcine Skin
2.2.7. Data Treatment and Statistical Analysis
3. Results and Discussions
3.1. Conversion of TBF Salt to the Base Form
3.2. TGA and DSC Analysis of TBF-Free Base
3.3. Solubility Parameters, Solubility, and Stability Studies
3.4. Dynamic Vapour Sorption Studies
3.5. In Vitro Finite Dose Neat Solvent and Commercial Formulation Efficacy in Porcine Skin
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rotta, I.; Fleith Otuki, M.; Cristina Conegero Sanches, A.; Januário Correr, C. Efficacy of topical antifungal drugs in different dermatomycoses: A systematic review with meta-analysis. Rev. Assoc. Med. Bras. 2012, 58, 308–318. [Google Scholar] [PubMed]
- Charles, A.J. Superficial cutaneous fungal infections in tropical countries. Dermatol. Ther. 2009, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Garber, G. An overview of fungal infections. Drugs 2001, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Cheng, T. Common Superficial Fungal Infections—A Short Review Diseases Caused by Dermatophytes. Med. Bull. 2010, 15, 23–27. [Google Scholar]
- Weinstein, A.; Berman, B. Topical treatment of common superficial tinea infections. Am. Fam. Physician 2002, 65, 2095–2102. [Google Scholar]
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. 2007, 18, CD001434. [Google Scholar] [CrossRef]
- Newland, J.G.; Abdel-Rahman, S.M. Update on terbinafine with a focus on dermatophytoses. Clin. Cosmet. Investig. Dermatol. 2009, 2, 49–63. [Google Scholar] [Green Version]
- Ballon, K.; Riebeshl, B.U.; Muller, B.W. Drug Liposome Partioning as a Tool for the Prediction of Human Passive Intestinal Absorption. Pharm. Res. 1999, 16, 882–888. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.J.; Wiseman, L.R.; Markham, A. Terbinafine An Update of its Use in Superficial Mycoses Karen. Drugs 1999, 58, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Ollert, M.; Abeck, D. German Collaborative Dermatophyte Drug Susceptibility Study Group Results of German multicenter study of antimicrobial susceptibilities of Trichophyton rubrum and Trichophyton mentagrophytes strains causing tinea unguium. Antimicrob. Agents Chemother. 1995, 39, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Ryder, N.S.; Favre, B. Antifungal activity and mechanism of action of terbinafine. Rev. Contemp. Pharmacother. 1997, 8, 275–288. [Google Scholar]
- Schuster, I.; Schaude, M.; Schatz, F.; Mieth, H. Preclinical characteristics of allylamines. In Sterol Biosynthesis Inhibitors: Pharmaceutical and Agrochemical Aspects; Ellis Horwood Ltd.: Chichester, UK, 1988; pp. 449–470. [Google Scholar]
- Schäfer-Korting, M.; Schoellmann, C.; Korting, H.C. Fungicidal activity plus reservoir effect allow short treatment courses with terbinafine in tinea pedis. Skin Pharmacol. Physiol. 2008, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liu, D.Z.; Liu, J.J.; Chang, T.W.; Ho, H.O.; Sheu, M.T. Development of terbinafine solid lipid nanoparticles as a topical delivery system. Int. J. Nanomed. 2012, 7, 4409–4418. [Google Scholar] [Green Version]
- Korting, H.C.; Kresimon, J.; Rychlik, R. Comparative evaluation of the activity and clinical effectiveness of terbinafine and bifonazole preparations in the treatment of pedal mycosis. Aktuelle Dermatol. 2004, 1, 210–217. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Grant, K. New Process for Preparing Terbinafine Free Base 2010. Patent KR 100979903B1, 3 September 2010. [Google Scholar]
- Parisi, N.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation and characterisation of hexamidine salts. Int. J. Pharm. 2015, 493, 404–411. [Google Scholar] [CrossRef] [Green Version]
- ICH Expert Working Group International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. In Validation of Analytical Procedures: Text and Methodology Q 2(R1); IFPMA: Geneva, Switzerland, 2005; pp. 6–13.
- Haque, T.; Crowther, J.M.; Lane, M.E.; Moore, D.J. Chemical ultraviolet absorbers topically applied in a skin barrier mimetic formulation remain in the outer stratum corneum of porcine skin. Int. J. Pharm. 2016, 510, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronaugh, R.L.; Stewart, R.F. Methods for In Vitro Percutaneous Absorption Studies 111: Hydrophobic Compounds. J. Pharm. Sci. 1983, 73, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Skelly, J.P.; Shah, V.P.; Maibach, H.I.; Guy, R.H.; Wester, R.C.; Flynn, G.; Yacobi, A. FDA and AAPS Report of the Workshop on Principles and Practices of In Vitro Percutaneous Penetration Studies: Relevance to Bioavailability and Bioequivalence. Pharm. Res. 1987, 4, 265–267. [Google Scholar] [CrossRef]
- OECD Organisation for Economic Cooperation and Development. Guidance Document for the Conduct of Skin Absorption Studies (Test no. 28); OECD: Paris, France, 2004; pp. 1–31. [Google Scholar]
- Castaldi, G.; Barreca, G.; Rossi, R. Process for the Preparation of Terbinafine. Patent US 6515181B2, 4 February 2003. [Google Scholar]
- Dias, M.; Hadgraft, J.; Lane, M.E. Influence of membrane–solvent–solute interactions on solute permeation in model membranes. Int. J. Pharm. 2006, 336, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.D. Using solubility parameters in cosmetics formulation. J. Soc. Cosmet. Chem. 1985, 333, 319–333. [Google Scholar]
- Paz-Alvarez, M.; Pudney, P.D.A.; Hadgraft, J.; Lane, M.E. Topical delivery of climbazole to mammalian skin. Int. J. Pharm. 2018, 549, 324–327. [Google Scholar] [CrossRef]
- Trottet, L.; Merly, C.; Mirza, M.; Hadgraft, J.; Davis, A.F. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. Int. J. Pharm. 2004, 274, 213–219. [Google Scholar] [CrossRef]
- Brinkmann, I.; Müller-Goymann, C.C. An attempt to clarify the influence of glycerol, propylene glycol, isopropyl myristate and a combination of propylene glycol and isopropyl myristate on human stratum corneum. Pharmazie 2005, 60, 215–220. [Google Scholar]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E.; Majella, E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef]
- Kasting, G.B.; Francis, W.R.; Roberts, G.E. Skin Penetration Enhancement of Triprolidine Base by Propylene Glycol. J. Pharm. Sci. 1993, 82, 551–552. [Google Scholar] [CrossRef]
- Pudney, P.D.A.; Mélot, M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. An in vivo confocal Raman study of the delivery of trans-retinol to the skin. Appl. Spectrosc. 2007, 61, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Liron, Z.; Cohen, S. Percutaneous absorption of alkanoic acids II: Application of regular solution theory. J. Pharm. Sci. 1984, 73, 538–542. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The role of vehicle interactions on permeation of an active through model membranes and human skin. Int. J. Cosmet. Sci. 2012, 34, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Chadha, G.; Sathigari, S.; Parsons, D.L.; Babu, R.J. In vitro percutaneous absorption of genistein from topical gels through human skin. Drug Dev. Ind. Pharm. 2011, 37, 498–505. [Google Scholar] [CrossRef]
- Gannu, R.; Vishnu, Y.; Kishan, V.; RaO, Y. In vitro permeation of carvedilol through porcine skin: Effect of vehicles and penetration enhancers. PDA J. Pharm. Sci. Technol. 2008, 62, 256–263. [Google Scholar]
- Brinkmann, I.; Müller-Goymann, C.C. Role of isopropyl myristate, isopropyl alcohol and a combination of both in hydrocortisone permeation across the human stratum corneum. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 393–404. [Google Scholar] [CrossRef]
- Dias, M.; Hadgraft, J.; Lane, M.E. Influence of membrane-solvent-solute interactions on solute permeation in skin. Int. J. Pharm. 2007, 340, 65–70. [Google Scholar] [CrossRef]
- Liu, P.; Bergstrom, T.K. Quantitative evaluation of aqueous isopropyl alcohol enhancement on skin flux of terbutaline (sulfate). 2. Permeability contributions of equilibrated drug species across human skin in vitro. J. Pharm. Sci. 1996, 85, 320–325. [Google Scholar] [CrossRef]
- Pendlington, R.U.; Whittle, E.; Robinson, J.A.; Howes, D. Fate of ethanol topically applied to skin. Food Chem. Toxicol. 2001, 39, 169–174. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, R.M.; Herkenne, C.; Guy, R.H.; Hadgraft, J.; Oliveira, G.; Lane, M.E. Influence of ethanol on the solubility, ionization and permeation characteristics of ibuprofen in silicone and human skin. Skin Pharmacol. Physiol. 2009, 22, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Parisi, N.; Paz-Alvarez, M.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Topical delivery of hexamidine. Int. J. Pharm. 2016, 506, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsumi, Y.; Yokoo, M.; Senda, H.; Kakehi, K. Therapeutic efficacy of topically applied KP-103 against experimental tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 2002, 46, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Yokoo, M.; Arika, T.; Yamaguchi, H. In vitro antifungal activity of KP-103, a novel triazole derivative, and its therapeutic efficacy against experimental plantar tinea pedis and cutaneous candidiasis in guinea pigs. Antimicrob. Agents Chemother. 2001, 45, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Sugiura, K.; Hashimoto, T.; Ueda, A.; Konno, Y.; Tatsumi, Y. Efficacy coefficients determined using nail permeability and antifungal activity in keratin-containing media are useful for predicting clinical efficacies of topical drugs for onychomycosis. PLoS ONE 2016, 11, e0159661. [Google Scholar] [CrossRef]
- Uchida, K.; Yamaguchi, H. Studies on the Affinity of Terbinafine with Keratine. Jpn. J. Med. Mycol. 1993, 34, 207–212. [Google Scholar] [CrossRef]
- Suhonen, T.M.; Bouwstra, J.A.; Urtti, A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 1999, 59, 149–161. [Google Scholar] [CrossRef]
- Dykes, P.; Thomas, R.; Lever, L.; Marks, R. Pharmacokinetics of topically applied terbinafine: Results from studies in healthy volunteer subjects and patients with pityriasis versicolor. J. Dermatolog. Treat. 1990, 1, 19–21. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.S.M.M.A.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. https://doi.org/10.3390/pharmaceutics11100548
Hossain ASMMA, Sil BC, Iliopoulos F, Lever R, Hadgraft J, Lane ME. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics. 2019; 11(10):548. https://doi.org/10.3390/pharmaceutics11100548
Chicago/Turabian StyleHossain, A. S. M. Monjur Al, Bruno C. Sil, Fotis Iliopoulos, Rebecca Lever, Jonathan Hadgraft, and Majella E. Lane. 2019. "Preparation, Characterisation, and Topical Delivery of Terbinafine" Pharmaceutics 11, no. 10: 548. https://doi.org/10.3390/pharmaceutics11100548
APA StyleHossain, A. S. M. M. A., Sil, B. C., Iliopoulos, F., Lever, R., Hadgraft, J., & Lane, M. E. (2019). Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics, 11(10), 548. https://doi.org/10.3390/pharmaceutics11100548