Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Miscibility and Stability Studies
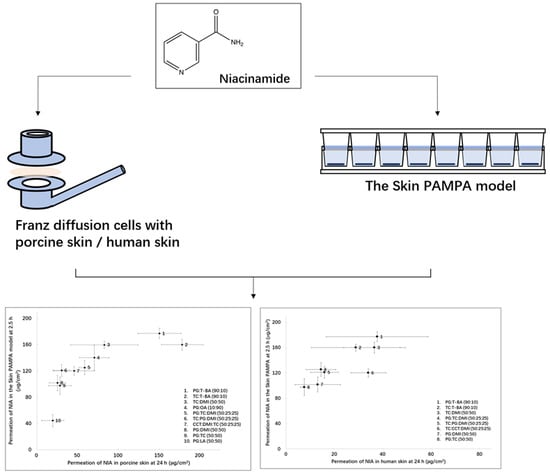
2.3. The Skin PAMPA Permeation Studies
2.4. Permeation Studies in Porcine and Human Skin
2.5. Data Analysis
3. Results and Discussion
3.1. Stability Determination
3.2. Skin PAMPA Permeation Studies
3.3. Porcine Skin Permeation and Mass Balance Studies
3.4. Human Skin Permeation and Mass Balance Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| NIA | Niacinamide |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| ANOVA | One-way analysis of variance |
| PG | Propylene glycol |
| DMI | Dimethyl isosorbide |
| OA | Oleic acid |
| LA | Linolenic acid |
| T-BA | t-butyl alcohol |
| TC | Transcutol® P |
| CCT | Caprylic/capric triglyceride |
| PEG | Polyethylene glycol |
| CIRP | Cosmetic Ingredient Review Panel |
| SD | Standard deviation |
| SC | Stratum corneum |
| NMR | Nuclear magnetic resonance |
| FTIR | Fourier transform infrared spectroscopy |
References
- Matts, P.; Oblong, J.; Bissett, D.L. A Review of the range of effects of niacinamide in human skin. Int. Fed. Soc. Cosmet. Chem. Mag. 2002, 5, 285–289. [Google Scholar]
- Wohlrab, J.; Kreft, D. Niacinamide-mechanisms of action and its topical use in dermatology. Ski. Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Lee, K.-K.; Park, M.-H.; Hyun, S.-S.; Kahn, S.-Y.; Joo, K.-S.; Kang, H.-C.; Kwon, W.-T. In vivo anti-melanogenesis activity and in vitro skin permeability of niacinamide-loaded flexible liposomes (Bounsphere™). J. Drug Deliv. Sci. Technol. 2016, 31, 147–152. [Google Scholar] [CrossRef]
- Papich, M.G. Niacinamide. In Saunders Handbook of Veterinary Drugs, 4th ed.; Saunders: St. Louis, MO, USA, 2016; pp. 562–563. [Google Scholar] [CrossRef]
- Mohammed, D.; Crowther, J.M.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Influence of niacinamide containing formulations on the molecular and biophysical properties of the stratum corneum. Int. J. Pharm. 2013, 441, 192–201. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- Parisi, N.; Matts, P.J.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation and characterisation of hexamidine salts. Int. J. Pharm. 2015, 493, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta—Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadgraft, J.; Lane, M.E. Advanced topical formulations (ATF). Int. J. Pharm. 2016, 514, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin II. Influence of binary and ternary solvent systems. Eur. J. Pharm. Sci. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Oxybutynin permeation in skin: The influence of drug and solvent activity. Int. J. Pharm. 2010, 384, 67–72. [Google Scholar] [CrossRef]
- Kung, C.-P.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Preparation, Characterization and Dermal Delivery of Methadone. Pharmaceutics 2019, 11, 509. [Google Scholar] [CrossRef] [Green Version]
- Haque, T.; Lane, M.E.; Sil, B.C.; Crowther, J.M.; Moore, D.J. In vitro permeation and disposition of niacinamide in silicone and porcine skin of skin barrier-mimetic formulations. Int. J. Pharm. 2017, 520, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Sil, B.C.; Moore, D.J.; Lane, M.E. Use of LC-MS analysis to elucidate by-products of niacinamide transformation following in vitro skin permeation studies. Int. J. Cosmet. Sci. 2018, 40, 525–529. [Google Scholar] [CrossRef] [Green Version]
- CIRP. Caprylic/Capric Triglyceride. Int. J. Toxicol. 2003, 22, 4–5. [Google Scholar] [CrossRef]
- Leopold, C.S.; Lippold, B.C. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC). J. Pharm. Pharmacol. 1995, 47, 276–281. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M.; Williams, A. Synergy Between Chemical Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 373–385. [Google Scholar] [CrossRef]
- van Zyl, L.; du Preez, J.; Gerber, M.; du Plessis, J.; Viljoen, J. Essential Fatty Acids as Transdermal Penetration Enhancers. J. Pharm. Sci. 2016, 105, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.D.; Björklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum—Relation between molecular effects and barrier function. J. Control. Release 2016, 232, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.H.; Srivilai, J.; Ibrahim, S.A.; Strasinger, C.; Hammell, D.C.; Hassan, H.E.; Stinchcomb, A.L. The Sensitivity of In Vitro Permeation Tests to Chemical Penetration Enhancer Concentration Changes in Fentanyl Transdermal Delivery Systems. AAPS Pharmscitech 2018, 19, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.P.; Scott, R.C. Pig ear skin as an in-vitro model for human skin permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Zhao, K.; Singh, J. In vitro permeability and binding of hydrocarbons in pig ear and huamn abdominal skin. Drug Chem. Toxicol. 2002, 25, 83–92. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal Models for Percutaneous Absorption. J. Appl. Toxicol. 2014, 35, 1–10. [Google Scholar] [CrossRef]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Li, S.K. Efficiency of Fatty Acids as Chemical Penetration Enhancers: Mechanisms and Structure Enhancement Relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M. Lateral chain packing in lipids and membranes. J. Lipid Res. 1984, 25, 1490–1500. [Google Scholar]






| Formulation | Percentage Permeation (%) at 2.5 h |
|---|---|
| PG:LA (50:50) | 26.9 ± 5.7 |
| PG:DMI (50:50) | 66.0 ± 6.9 |
| PG:TC (50:50) | 68.0 ± 12.3 |
| PG:OA (10:90) | 95.2 ± 13.8 |
| PG:T-BA (90:10) | 103.7 ± 0.5 |
| TC:T-BA (90:10) | 97.3 ± 5.1 |
| TC:DMI (50:50) | 97.2 ± 3.3 |
| TC:PG:DMI (50:25:25) | 77.5 ± 8.2 |
| TC:CCT:DMI (50:25:25) | 75.3 ± 4.9 |
| TC:CCT:DMI (50:25:25) | 81.2 ± 3.2 |
| Formulation | Washing % | Extraction % | Permeation % | Total % |
|---|---|---|---|---|
| PG:DMI (50:50) | 47.1 ± 5.4 | 22.9 ± 13.2 | 11.5 ± 3.9 | 81.5 ± 5.6 |
| PG:OA (10:90) | 37.1 ± 5.4 | 9.6 ± 1.0 | 30.2 ± 7.2 | 76.8 ± 5.9 |
| PG:TC (50:50) | 43.4 ± 3.9 | 23.4 ± 5.6 | 13.4 ± 6.9 | 80.2 ± 6.7 |
| PG:LA (50:50) | 55.6 ± 12.3 | 20.6 ± 4.4 | 9.2 ± 6.6 | 85.4 ± 7.9 |
| PG:T-BA (90:10) | 3.5 ± 0.8 | 10.1 ± 7.6 | 71.3 ± 12.6 | 84.9 ± 7.1 |
| TC:T-BA (90:10) | 6.1 ± 5.2 | 15.3 ± 11.9 | 79.5 ± 12.0 | 100.9 ± 13.8 |
| TC:DMI (50:50) | 35.3 ± 13.6 | 10.7 ± 3.5 | 35.2 ± 16.0 | 81.2 ± 1.8 |
| PG:TC:DMI (50:25:25) | 36.1 ± 8.5 | 18.8 ± 8.8 | 25.1 ± 3.8 | 80.1 ± 9.7 |
| TC:CCT:DMI (50:25:25) | 39.8 ± 15.1 | 28.0 ± 12.8 | 18.2 ± 7.6 | 86.0 ± 8.9 |
| TC:PG:DMI (50:25:25) | 43.2 ± 4.0 | 32.9 ± 13.8 | 13.2 ± 1.9 | 89.2 ± 16.8 |
| Formulations | Washing% | Extraction% | Permeation% | Total% |
|---|---|---|---|---|
| PG:DMI (50:50) | 59.6 ± 7.1 | 17.4 ± 3.9 | 5.6 ± 4.0 | 82.6 ± 7.8 |
| PG:OA (10:90) | 37.7 ± 6.7 | 9.2 ± 0.6 | 40.7 ± 6.5 | 87.6 ± 0.8 |
| PG:TC (50:50) | 69.8 ± 7.8 | 29.0 ± 18.5 | 4.4 ± 2.3 | 103.1 ± 13.4 |
| PG:LA (50:50) | 27.1 ± 5.6 | 3.4 ± 2.0 | 46.1 ± 5.1 | 76.5 ± 5.1 |
| PG:T-BA (90:10) | 52.8 ± 9.0 | 20.1 ± 6.0 | 12.3 ± 4.2 | 85.2 ± 10.9 |
| TC:T-BA (90:10) | 39.8 ± 16.5 | 34.7 ± 10.5 | 15.6 ± 5.6 | 90.1 ± 5.2 |
| TC:DMI (50:50) | 75.1 ± 11.7 | 13.2 ± 4.7 | 10.2 ± 4.4 | 98.5 ± 11.7 |
| PG:TC:DMI (50:25:25) | 64.6 ± 4.5 | 12.2 ± 2.0 | 6.2 ± 2.3 | 83.0 ± 4.2 |
| TC:CCT:DMI (50:25:25) | 44.9 ± 6.0 | 29.7 ± 4.9 | 12.9 ± 3.3 | 87.5 ± 4.9 |
| TC:PG:DMI (50:25:25) | 66.5 ± 9.1 | 14.6 ± 3.4 | 6.6 ± 2.5 | 87.8 ± 6.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kung, C.-P.; Sil, B.C.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Sinko, B. Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems. Pharmaceutics 2019, 11, 668. https://doi.org/10.3390/pharmaceutics11120668
Zhang Y, Kung C-P, Sil BC, Lane ME, Hadgraft J, Heinrich M, Sinko B. Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems. Pharmaceutics. 2019; 11(12):668. https://doi.org/10.3390/pharmaceutics11120668
Chicago/Turabian StyleZhang, Yanling, Chin-Ping Kung, Bruno C. Sil, Majella E. Lane, Jonathan Hadgraft, Michael Heinrich, and Balint Sinko. 2019. "Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems" Pharmaceutics 11, no. 12: 668. https://doi.org/10.3390/pharmaceutics11120668
APA StyleZhang, Y., Kung, C. -P., Sil, B. C., Lane, M. E., Hadgraft, J., Heinrich, M., & Sinko, B. (2019). Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems. Pharmaceutics, 11(12), 668. https://doi.org/10.3390/pharmaceutics11120668