1. Introduction
The fabrication of innovative devices with peculiar structural properties is raising a lot of interest with regard to the controlled release of bioactive molecules. Micro-sized capsules have been used in cancer therapy as depots to encapsulate anti-cancer agents [
1], or more recently, as micro-scaffolds loaded with multiple agents to stimulate specific signaling pathways and instruct cellular responses in simulated biological micro-environments [
2]. In all of these cases, an accurate design of polymer matrix properties is required to optimize the encapsulation of drugs or actives, to protect labile molecules from hazardous environmental conditions, and to define a tailored release profile as a function of the specific application. Accordingly, Dawes et al. have demonstrated that the release profile can be drastically influenced by the spatial distribution of molecules, strictly related to the size of microspheres [
3]. Moreover, the effect of peculiar chemical and physical properties of carriers may be corroborated by the structural properties at the micro/sub-micrometric level, thus finely tuning the release profile by the control of the relative diffusion/degradation mechanisms [
4,
5].
In this context, core/shell structures able to confine drugs preferentially in a core region may allow fine control of the water/drug exchange via diffusion, as a function of the peculiar properties of the inner/outer phases, thereby influencing burst release extension [
6]. Meanwhile, an accurate modification of the shell properties—i.e., thickness—may also contribute to define the active transport kinetics [
7]. In the last decade, several technologies have been explored to design micro- and nano-structured platforms for biomedical use. Among them, electrohydrodynamic atomization (EHDA) identifies one of the most powerful bottom-up technologies rapidly emerging as promising and innovative tools for the development of micro/sub-micro-vectors for molecular and cell delivery [
8]. The fundamental working principle is based on the capability to break up a complex fluid (i.e., viscous polymeric fluids, inorganic gels, or composite slurries) into charged droplets by means of electrostatic forces [
9]. Through a careful optimization of process parameters and an appropriate definition of the atomization setup—depending on the peculiar properties of nozzle, needle, and collectors—complex active micro- and nanostructures may be variously engineered to face more relevant key challenges of healthcare (e.g., advanced chemotherapy, biomedical diagnostics, and tissue regeneration). In contrast with other atomization techniques (i.e., gas atomization [
10], vacuum atomization [
11], centrifugal atomization [
12], rotating disk atomization [
13], ultrasonic atomization [
14]), EHDA has some relevant advantages, including relative ease of droplet generation, great control of droplet transport, ability to avoid coalescence of droplets due to an electric charge of the same polarity on the droplets, enhanced adhesion and deposition, and so on [
15].
Herein, we propose the fabrication of mono-component devices made of cellulose acetate by a modified process configuration, based on the use of coaxial needles to design core/shell architectures able to confine anti-inflammatory drugs more efficiently for oral delivery applications.
3. Results and Discussion
In the last decade, several studies have been focused on the design of drug delivery systems to finely control release kinetics for a site-specific delivery, in order to reduce side effects and improve therapeutic efficacy and safety. In this work, new cellulose-based carriers were engineered via electrofluidodynamic techniques to achieve a sustained release of KL for several hours after the in vitro administration. In more detail, two different systems—MC and BC respectively—were fabricated via a mechanism of fluid breaking into micro-droplets driven by the application of electrostatic forces, recently defined as electro hydrodynamic atomization [
20]. The term “electro” refers to the application of electrostatic forces to promote solution dropping, the term “hydrodynamic” refers to the fluid dynamic conditions applied to the viscous polymer solutions, and “atomization” refers to the capability of breaking a bulk liquid droplet into finely dispersed micro-droplets. This bottom-up technology is particularly suitable for synthesizing microstructures in the form of particles/capsules with different sizes and shapes, as a function of the capability of high-voltage electric forces to interact with and manipulate viscoelastic polymer solutions.
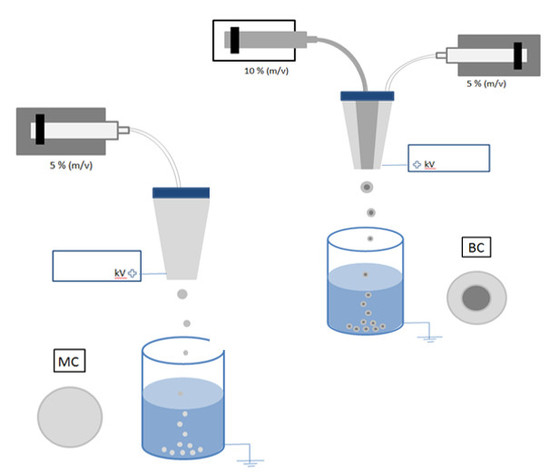
A conventional EHDA process for the fabrication of MC systems is schematized in
Figure 1A. Under the application of electrostatic forces over the voltage threshold, the surface tension of CA solution was rapidly overcome, thus promoting the formation of a relatively monodispersed size distribution of droplets. Once collected into the SDS-rich water bath, particles with round-like shapes (
Figure 1B) were forming, due to the ionic interactions of the CA solution with SDS and water into the coagulation bath. In particular, the diffusion of water into CA micro-droplets drastically reduced acetone concentration by a thermodynamically driven stripping mechanism, which promotes the fast precipitation of CA microcapsules. This phenomenon was also controlled by the local interaction between CA and SDS macromolecules, which concurred to stabilize particle shape during the acetone removal. Hence, CA microcapsules with a narrow size distribution were obtained (
Figure 1B). It is noteworthy that no relevant modification of physical properties of CA capsules was recognized after the process, as confirmed by thermal analyses (
Figure 1C): weight loss and thermal flow with respect to the temperature were comparable with those from the net CA, as reported elsewhere [
21]. According to previous works, the first inflection point on the TGA signal (
Figure 1C, black line) at temperature lower than 100 °C, was probably due to a weight loss (about 3%) caused by the evaporation of residual humidity. On the contrary, at around 360 °C, a substantial weight loss due to polymer degradation was noticeable. At the same temperature, on the DSC signal (
Figure 1C, in red), the positive peak revealed the corresponding rise of the thermal flow due to the polymer degradation [
22].
In contrast, EHDA process conditions may drastically influence the morphology of MC capsules in terms of size and shape. In
Figure 2A–C, a summary of the morphological characterization performed via optical microscopy supported by image analysis is reported to identify the best processing conditions (
V,
Q) to fabricate round-like capsules. Different sets of MC carriers were fabricated by singularly varying voltage from 12 to 21 kV, and flow rate from 1.5 to 2.5 mL/h.
The capsule shape, slightly elliptical, was initially approximated to a rounded sphere to measure the average diameter by image analysis software. The elaboration of optical images via dedicated software clearly shows a large variability in the average size, from 1248 ± 120 µm down to 287 ± 76 µm, underlining a strict size distribution in the case of lower applied voltage values (
Figure 2B). As expected, as the flow rate increased, a significant increase in capsule size was recorded. However, by the application of higher voltage values from 18 kV, mainly predominant instability phenomena were recognized, with evidence of more remarkable heterogeneities in size causing the formation of two populations of capsules with different sizes (see the bi-modal size distribution measured in
Figure 2C.) It is noteworthy that a slightly elliptical shape of CA capsules—less than 10%—was revealed by optical images; this effect was mainly due to the droplet elongation occurring into the coagulation bath, prior to the droplet stabilization. An accurate image elaboration was addressed to evaluate the effect of singular processing parameters on the singular capsule shape.
In particular, for each process condition—i.e., for each value of the imposed flow rate
Q and tension
V—a number of capsules ranging between 60 and 140 was analyzed, by measuring the mayor and minor axis (a and b in
Figure 3A). Assuming each capsule was a rotational ellipsoid, symmetric respect to its major axis
a, it was possible to calculate each capsule’s volume and external surface. In
Figure 3B and
Figure 3C, surface and volume are reported as a function of the voltage V, with parametric respect to the imposed flow rate
Q. The average of the geometric quantity was measured over the entire sample population, and the standard error of the mean as an error bar was reported (see the lines indicated as guides for the eyes). As previously observed, capsule size is a decreasing function of the voltage, while in the range of parameters here investigated the influence of the flow rate on the size is not monotonic. The shape of CA capsules was also quantified. In
Figure 3D, the deformation parameter
D is reported as a function of the voltage, parametric in the flow rate. Finally, in
Figure 3E the surface/volume ratio is also reported. A significant influence of the voltage V on this parameter was observed. The three data series, independently from the value of the imposed flow rate, have been fit to a polynomial curve, reported as a dashed line. This effect may be considered directly ascribable to the peculiar viscoelastic properties of the polymer solution, which is able to affect the droplet shape by the elastic deformation of the polymer solution at the tip of the needle, imparting an elliptical shape to the droplets’. In order to better control the shape, polymer solutions with different viscosities have been investigated in past literature [
23]. Hence, two solutions with different viscosities have been used for the fabrication of biphasic CA capsules. This has allowed the containment of capsule elongation, also influencing specific functionalities for a controlled release of anti-inflammatory drugs. Recent studies have demonstrated the versatility and high feasibility of the EHDA process to particularize properties and functions of micro-sized devices by the easy customization of the process setup configuration, in order to obtain tailored systems for different applications in biomedical areas (i.e., regenerative medicine, drug delivery, bio-cosmetics, bio-packaging) [
17,
24] that are easily scalable for industrial production [
25].
In
Figure 4A, the scheme of BC preparation was reported. In this case, the use of coaxial needles enabled simultaneously processing of two solutions with peculiar chemical/physical properties, thus imparting to the micro-carrier a core–shell structure [
26], suitable for targeting and controlling the release of drugs and molecular species. Herein, two different CA solutions with different concentrations, 5%
w/
v (outlet) and 10%
w/
v (inlet) were used to generate microcapsules—i.e., only made of CA but with core shell architecture--to gradually release KL. SEM images (
Figure 4B) show a zoom of a BC cross section, to qualitatively investigate its highly heterogeneous inner structure. In particular, a highly porous interconnected matrix was recognized at the outer regions. This is basically ascribable to the effect of local interactions occurring at the interface of the droplet, where a less concentrated CA solution promotes the diffusion of the surrounding coagulation medium and a gradual exchange of acetone at equilibrium. Meanwhile, a skin effect was also detected, probably due to the contribution of SDS concurrent to the surface stabilization. Notably, this peculiar morphology did not compromise the capability to entrap bioactive molecules. Indeed, the discontinuity in viscosity (
Figure 4C) between the inner and the outer CA solution assures the creation of a stable interface that is better able to confine drugs into the inner and more viscous core of capsule, preventing massive and uncontrolled drug loss towards the external regions. As a consequence, the encapsulation efficiency of BC was about twice that of MC. An accurate investigation of the rheological properties was assessed onto CA solutions, in order to correlate the contribution of the relative viscosity to the final properties of MC and BC capsules in terms of drug release. The relationship between viscosity and shear rate was primarily evaluated, with shear rate varying in the range of 1–100 Hz. The results, shown in
Figure 4C, clearly demonstrate a significant difference in viscosity, with typical shear-thinning behavior [
27,
28] independent from the solution concentration. At the beginning, the increment in the viscosity curve of outer CA solution was related to an initial inertia, which was mainly detectable in the case of higher concentrations. On the contrary, such an increment was not visible in the curve of the less concentrated solution, where signal noises tended to cover lower viscosity values. In the latter case, the values were closer to the lowest sensitivity of the transducer.
The rheological characterization was carrying out by analyzing the time-dependent viscosity of the two differently concentrated solutions at 1 and 5 Hz (
Figure 5A,B). Two minutes was the chosen time test, in order to limit the effects of solvent evaporation. As demonstrated by
Figure 4 and
Figure 5A,B, the viscosity of the two solutions decreases by increasing both shear rate and time. As expected, the viscosity values in the case of more concentrated solution (
Figure 5A) are always higher than the corresponding ones in the case of less concentrated solution (
Figure 5B).
The characterization was finally addressed to the analysis of viscoelastic properties. Storage and loss moduli (G’ and G’’) were evaluated in the function of frequency by means of a frequency sweep test, with a strain value equal to 0.0025, falling into the linear range of both moduli. The results shown in
Figure 5 indicate that in the case of 10% concentrated solution (c), at lower frequency values characterizing the initial part of the test, the storage (G’) and viscous (G’’) components of the solution are similar. Increasing the frequency makes G’ become clearly separated from G’’, showing the higher elastic response of the polymer. In that case, the elastic property of the solution is dominant, indicating that the formation of an organogel-like structure [
29]. This behavior is not noticeable in the 5% concentrated solution (
Figure 5D), probably due to the lower concentration of the polymer.
Hence, the morphological features of MC and BC capsules selected for the in vitro release tests are summarized (
Table 1). The presence of a more viscous inner phase promotes an increase of particle size but a decrease of the deformation parameter (
D), thus stabilizing the shape of capsules in the rounded form.
KL in vitro release in simulated biological fluids of the gastro-intestinal tract was also investigated, in order to underline the main differences due to the presence of the core/shell architecture. Release profiles of MC and BC carriers are reported in
Figure 6A,B.
At pH 1.2, a significant reduction of the percentage of the released drug was recorded, from 18% in the case of BC—to 26% in the case of MC. This result was strictly related to the higher capability of bi-phasic capsules with peculiar core/shell architecture. Indeed, the more viscous core of BC allowed a more efficient KL confinement with respect to MC, thus playing not only a storing role but also a protective function of inner bioactive molecules. Accordingly, at pH 6.8, a total amount of KL equal to 82% and 74% was recorded for BC and MC, respectively (
Figure 6A). Moreover, a comparative evaluation of in vitro release profiles was also conducted in SIF (pH 6.8) until 6 h. Different KL release profiles were detected as a function of the peculiar architecture of the compared devices (
Figure 6B). In the case of BC, with a core/shell structure, the fluid transport in the inner core, which is denser and more viscous then the outer shell, was partially hindered, and consequently, KL release was poorly delayed. In contrast, in the case of MC, with a homogeneous and less viscous body, the transport of KL was facilitated and KL was released more rapidly. During the first hours, a sustained release was reported, mainly driven by the presence of higher KL gradients. In particular, a significant discrepancy between BC and MC profiles was observed mainly until 3 h, with a gap of released KL of about 15–20% over the same time (
Figure 6B). This peculiar release profile is promising for designing smart micro-vectors able to locally guide drug administration by the controlled swelling/adsorption properties of polymer matrices in response to microenvironmental stimuli, such as pH.
Hence, the use of core shell architecture in CA capsules, developed via EHDA, may be efficacious for controlling the release mechanism of drugs, if compared with other processing techniques (i.e., emulsion-solvent evaporation [
30], the nanoprecipitation method [
31], and the supercritical anti-solvent process [
32]. Despite the total amount of drug that was released over the same time—from 6 to 8 h—the possibility of encapsulating the drugs into a more viscous solution, with respect to those conventionally used in other processes, allows the delivery of the active principle more slowly immediately after the administration, thus reducing the side effects due to the presence of a more pronounced burst release [
33]. More interestingly, this mechanism could be also customized, to some extent, by appropriately varying the viscosity of the core solution, thereby giving the opportunity to design different BC systems with tailored release profiles for the selected administration of different drugs (i.e., anti-inflammatory, antibiotics, etc.) as a function of target demands.