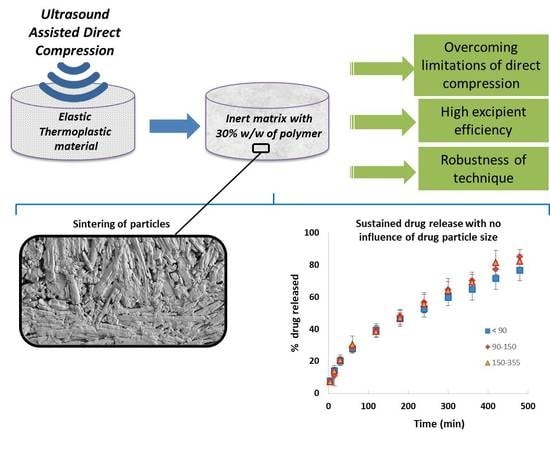
Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Blends Preparation
2.2.2. Preparation of TPU Tablets by Direct Compression
2.2.3. Preparation of TPU Tablets by Ultrasound-Assisted Direct Compression
2.2.4. Physical Characterization of TPU Tablets
2.2.5. Dissolution Testing of TPU Tablets and Modelling
2.2.6. Mercury Porosimetry Measurements
2.2.7. Measurement of Fractal Dimension
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Estimation of the Excipient Efficiency
3. Results and Discussion
3.1. Characterization of TPU Tablets Obtained by USAC
3.2. Dissolution Testing of TPU Tablets and Modelling
3.3. Mercury Porosimetry Measurements
3.4. Measurement of Fractal Dimension
3.5. Scanning Electron Microscopy (SEM)
3.6. Estimation of the Excipient Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galdón, E.; Casas, M.; Gayango, M.; Caraballo, I. First study of the evolution of the SeDeM expert system parameters based on percolation theory: Monitoring of their critical behavior. Eur. J. Pharm. Biopharm. 2016, 109, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Millán-Jiménez, M.; Galdón, E.; Ferrero, C.; Caraballo, I. Application of ultrasound-assisted compression in pharmaceutical technology. Design and optimization of oral sustained-release dosage forms. J. Drug Deliv. Sci. Technol. 2017, 42, 119–125. [Google Scholar] [CrossRef]
- Fini, A.; Holgado, M.A.; Rodriguez, L.; Cavallari, C. Ultrasound-compacted indomethacin/polyvinylpyrrolidone systems: Effect of compaction process on particle morphology and dissolution behavior. J. Pharm. Sci. 2002, 91, 1880–1890. [Google Scholar] [CrossRef]
- Fini, A.; Cavallari, C.; Ospitali, F. Effect of Ultrasound on the Compaction of Ibuprofen/Isomalt Systems. Pharmaceutics 2009, 1, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Levina, M.; Rubinstein, M.H.; Rajabi-Siahboomi, A.R. Principles and application of ultrasound in pharmaceutical powder compression. Pharm. Res. 2000, 17, 257–265. [Google Scholar] [CrossRef]
- Aguilar-De-Leyva, A.; Goncalves-Araujo, T.; Daza, V.; Caraballo, I. A new deferiprone controlled release system obtained by ultrasound-assisted compression. Pharm. Dev. Technol. 2014, 19, 728–734. [Google Scholar] [CrossRef]
- Caraballo, I.; Millán, M.; Fini, A.; Rodriguez, L.; Cavallari, C. Percolation thresholds in ultrasound compacted tablets. J. Control. Release 2000, 69, 345–355. [Google Scholar] [CrossRef]
- Levina, M.; Rubinstein, M.H. The Effect of Ultrasonic Vibration on the Compaction Characteristics of Paracetamol. J. Pharm. Sci. 2000, 89, 705–723. [Google Scholar] [CrossRef]
- Millán, M.; Caraballo, I. Effect of drug particle size in ultrasound compacted tablets. Continuum percolation model approach. Int. J. Pharm. 2006, 310, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sancin, P.; Caputo, O.; Cavallari, C.; Passerini, N.; Rodriguez, L.; Cini, M.; Fini, A. Effects of ultrasound-assisted compaction on Ketoprofen/Eudragit® S100 mixtures. Eur. J. Pharm. Sci. 1999, 7, 207–213. [Google Scholar] [CrossRef]
- Claeys, B.; Vervaeck, A.; Hillewaere, X.K.D.; Possemiers, S.; Hansen, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Eur. J. Pharm. Biopharm. 2015, 90, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Campiñez, M.D.; Benito, E.; Romero-Azogil, L.; Aguilar-de-Leyva, Á.; de Gracia García-Martín, M.; Galbis, J.A.; Caraballo, I. Development and characterization of new functionalized polyurethanes for sustained and site-specific drug release in the gastrointestinal tract. Eur. J. Pharm. Sci. 2017, 100, 285–295. [Google Scholar] [CrossRef]
- Markl, D.; Strobel, A.; Schlossnikl, R.; Bøtker, J.; Bawuah, P.; Ridgway, C.; Rantanen, J.; Rades, T.; Gane, P.; Peiponen, K.E.; et al. Characterisation of pore structures of pharmaceutical tablets: A review. Int. J. Pharm. 2018, 538, 188–214. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Aguilar-de-Leyva, Á.; Caraballo, I. Towards a rational basis for selection of excipients: Excipient Efficiency for controlled release. Int. J. Pharm. 2015, 494, 288–295. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Bonny, J.; Leuenberger, H. Determination of fractal dimensions of matrix-type solid dosage forms and their relation with drug dissolution kinetics. Eur. J. Pharm. Biopharm. 1993, 39, 31–37. [Google Scholar]
- Usteri, M.; Bonny, J.; Leuenberger, H. Fractal dimension of porous solid dosage forms. Pharm. Acta Helv. 1990, 65, 55–61. [Google Scholar]
- Aguilar-de-Leyva, Á.; Campiñez, M.D.; Casas, M.; Caraballo, I. Design space and critical points in solid dosage forms. J. Drug Deliv. Sci. Technol. 2017, 42, 134–143. [Google Scholar] [CrossRef]
- Leuenberger, H.; Rohera, B.; Haas, C. Percolation theory - a novel approach to solid dosage form design. Int. J. Pharm. 1987, 38, 109–115. [Google Scholar] [CrossRef]
- Caraballo, I.; Fernández-Arévalo, M.; Holgado, M.A.; Rabasco, A.M. Percolation theory: Application to the study of the release behaviour from inert matrix systems. Int. J. Pharm. 1993, 96, 175–181. [Google Scholar] [CrossRef]
- Caraballo, I.; Millán, M.; Rabasco, A.M. Relationship between drug percolation threshold and particle size in matrix tablets. Pharm. Res. 1996, 13, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, C.; Bawuah, P.; Markl, D.; Zeitler, J.A.; Ketolainen, J.; Peiponen, K.E.; Gane, P. On the role of API in determining porosity, pore structure and bulk modulus of the skeletal material in pharmaceutical tablets formed with MCC as sole excipient. Int. J. Pharm. 2017, 526, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamud, M.M.; Novo, M.F. The use of fractal analysis in the textural characterization of coals. Fuel 2008, 87, 222–231. [Google Scholar] [CrossRef]
- Pippa, N.; Dokoumetzidis, A.; Demetzos, C.; Macheras, P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: A review. Int. J. Pharm. 2013, 456, 340–352. [Google Scholar] [CrossRef]
- Xianwen Shen; Longjian Li; Wenzhi Cui; Ya Feng Improvement of fractal model for porosity and permeability in porous materials. Int. J. Heat Mass Transf. 2018, 121, 1307–1315. [CrossRef]
- Rodriguez, L.; Cini, M.; Cavallari, C.; Passerini, N.; Fabrizio Saettone, M.; Fini, A.; Caputo, O. Evaluation of theophylline tablets compacted by means of a novel ultrasound-assisted apparatus. Int. J. Pharm. 1998, 170, 201–208. [Google Scholar] [CrossRef]





| TPU Tablets with Different Drug Particle Size (μm) | Higuchi | Korsmeyer | Peppas & Sahlin | ||||
|---|---|---|---|---|---|---|---|
| b (min−0.5) | r2 | n | r2 | kd (min−0.5) | kr (min−1) | r2 | |
| <90 | 0.0339 | 0.9986 | 0.4915 | 0.9988 | 0.0387 | −2.10−4 | 0.9994 |
| 90–150 | 0.0373 | 0.9966 | 0.5366 | 0.9896 | 0.0361 | 9.10−5 | 0.9974 |
| 150–355 | 0.0354 | 0.9947 | 0.513 | 0.9938 | 0.0361 | −2.10−6 | 0.9957 |
| Drug Fraction | TPU Matrices | Porosity (%) | Median Pore Diameter (μm) | Dv (and range in μm) |
| <90 μm | 17.9 ± 0.7 | 2.1 | 2.883 (3.2–1.1) | |
| 90–150 μm | 19.8 ± 2.9 | 1.8 | 2.882 (2.5–1.1) | |
| 150–355 μm | 17.1 ± 1.9 | 1.7 | 2.899 (2.5–0.8) | |
| Drug Fraction | TPU Matrices after Dissolution Testing | |||
| <90 μm | 59.1 ± 0.4 | 33.7 | 2.9203 (17.2–90.6) | |
| 90–150 μm | 58.6 ± 1.8 | 41.1 | 2.9344 (21.3–90.3) | |
| 150–355 μm | 60.3 ± 0.1 | 40.5 | 2.9542 (13.9–90.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdón, E.; Casas, M.; Caraballo, I. Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression. Pharmaceutics 2019, 11, 157. https://doi.org/10.3390/pharmaceutics11040157
Galdón E, Casas M, Caraballo I. Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression. Pharmaceutics. 2019; 11(4):157. https://doi.org/10.3390/pharmaceutics11040157
Chicago/Turabian StyleGaldón, Eduardo, Marta Casas, and Isidoro Caraballo. 2019. "Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression" Pharmaceutics 11, no. 4: 157. https://doi.org/10.3390/pharmaceutics11040157
APA StyleGaldón, E., Casas, M., & Caraballo, I. (2019). Achieving High Excipient Efficiency with Elastic Thermoplastic Polyurethane by Ultrasound Assisted Direct Compression. Pharmaceutics, 11(4), 157. https://doi.org/10.3390/pharmaceutics11040157