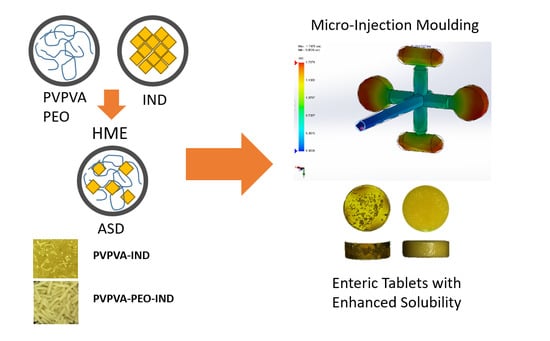
Micro-Injection Moulding of Poly(vinylpyrrolidone-vinyl acetate) Binary and Ternary Amorphous Solid Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry
2.2.2. Hot-Melt Extrusion
2.2.3. Micro-Injection Moulding
2.2.4. Morphology Analysis
2.2.5. Attenuated Total Reflectance Fourier Transformation Infrared Spectroscopy (ATR–FTIR)
2.2.6. Dissolution Studies
2.2.7. High-Performance Liquid Chromatography
3. Results and Discussion
3.1. Design of the Tablet Injection Mould
3.2. Micro-Injection Moulding of Tablets
3.3. Morphology Analysis
3.4. Thermal Analysis
3.5. Investigation of Intermolecular Interactions
3.6. Dissolution Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Kumar, S.; Mcginity, J.W. Pharmacy pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O., III. Challenges and strategies in thermal processing of amorphous solid dispersions: A review. AAPS PharmSciTech 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef]
- Démuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M.E. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Verstraete, G.; Mertens, P.; Grymonpré, W.; Van Bockstal, P.J.; De Beer, T.; Boone, M.N.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. A comparative study between melt granulation/compression and hot melt extrusion/injection molding for the manufacturing of oral sustained release thermoplastic polyurethane matrices. Int. J. Pharm. 2016, 513, 602–611. [Google Scholar] [CrossRef]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Injection molding and its application to drug delivery. J. Control. Release 2012, 159, 324–331. [Google Scholar] [CrossRef]
- Chatterjee, S. FDA perspective on continuous manufacturing. In Proceedings of the IFPAC Annual Meeting, Baltimore, MD, USA, 22–25 January 2012; pp. 34–42. [Google Scholar]
- Qi, S.; Craig, D. Recent developments in micro- and nanofabrication techniques for the preparation of amorphous pharmaceutical dosage forms. Adv. Drug Deliv. Rev. 2016, 100, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Quinten, T.; De Beer, T.; Vervaet, C.; Remon, J.P. Evaluation of injection moulding as a pharmaceutical technology to produce matrix tablets. Eur. J. Pharm. Biopharm. 2009, 71, 145–154. [Google Scholar] [CrossRef]
- Giboz, J.; Copponnex, T.; Mélé, P. Microinjection molding of thermoplastic polymers: A review. J. Micromechanics Microengineering 2007, 17, R96–R109. [Google Scholar] [CrossRef]
- Pezzoli, R.; Lyons, J.G.; Gately, N.; Higginbotham, C.L. Investigation of miscibility estimation methods between indomethacin and poly (vinylpyrrolidone-co-vinyl acetate). Int. J. Pharm. 2018, 549, 50–57. [Google Scholar] [CrossRef]
- Pezzoli, R.; Lyons, J.G.; Gately, N.; Higginbotham, C.L. Stability studies of hot-melt extruded ternary solid dispersions of poorly-water soluble indomethacin with poly(vinyl pyrrolidone-co-vinyl acetate) and polyethylene oxide. J. Drug Deliv. Sci. Technol. 2019, 52, 248–254. [Google Scholar] [CrossRef]
- U. S. Pharmacopeia Buffer Solutions. Available online: http://www.uspbpep.com/usp29/v29240/usp29nf24s0_ris1s119.html (accessed on 22 April 2019).
- Postawa, P.; Koszkul, J. Change in injection moulded parts shrinkage and weight as a function of processing conditions. J. Mater. Process. Technol. 2005, 162–163, 109–115. [Google Scholar] [CrossRef]
- Sha, B.; Dimov, S.; Griffiths, C.; Packianather, M.S. Investigation of micro-injection moulding: Factors affecting the replication quality. J. Mater. Process. Technol. 2007, 183, 284–296. [Google Scholar] [CrossRef]
- Hassan, H. An experimental work on the effect of injection molding parameters on the cavity pressure and product weight. Int. J. Adv. Manuf. Technol. 2013, 67, 675–686. [Google Scholar] [CrossRef]
- U. S. Pharmacopeia Indomethacin Extended-Release Capsules. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/indomethacin-er-capsules-rb-notice.pdf (accessed on 16 May 2019).
- Eggenreich, K.; Windhab, S.; Schrank, S.; Treffer, D.; Juster, H.; Steinbichler, G.; Laske, S.; Koscher, G.; Roblegg, E.; Khinast, J.G. Injection molding as a one-step process for the direct production of pharmaceutical dosage forms from primary powders. Int. J. Pharm. 2016, 505, 341–351. [Google Scholar] [CrossRef]
- McCarthy, E.; Johnston, S.; Barsom, M.; Dinunzio, J.; Lowell, M. Melt extrusion and injection molding for the development of pharmaceutical mini-tablets—Case study using clotrimazole. In Proceedings of the SPE ANTEC, Anaheim, CA, USA, 8–10 May 2017; pp. 1836–1839. [Google Scholar]
- Nyquist, R.A. Infrared studies of ketones. Parameters affecting the induced carbonyl stretching vibration by solute/solvent interaction. Appl. Spectrosc. 1990, 44, 433–438. [Google Scholar] [CrossRef]
- Taylor, L.S.; Langkilde, F.W.; Zografi, G. Fourier transform raman spectroscopic study of the interaction of water vapor with amorphous polymers. J. Pharm. Sci. 2001, 90, 888–901. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 1997, 14, 1691–1698. [Google Scholar] [CrossRef]
- Yuan, X.; Xiang, T.X.; Anderson, B.D.; Munson, E.J. Hydrogen bonding interactions in amorphous indomethacin and its amorphous solid dispersions with poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-vinyl acetate) studied using 13C solid-state NMR. Mol. Pharm. 2015, 12, 4518–4528. [Google Scholar] [CrossRef]
- Tres, F.; Treacher, K.; Booth, J.; Hughes, L.P.; Wren, S.A.C.; Aylott, J.W.; Burley, J.C. Indomethacin-kollidon VA64 extrudates: A mechanistic study of pH-dependent controlled release. Mol. Pharm. 2016, 13, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.L.; Paradkar, A.; Deshmukh, S.; Booth, J. Injection moulding of controlled release solid dispersions. In Proceedings of the Controlled Release Society Meetings, Edinburgh, Scotland, UK, 26–29 July 2015. [Google Scholar]
- Hörter, D.; Dressman, J.B. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 1997, 25, 3–14. [Google Scholar] [CrossRef]
- Cantin, O.; Siepmann, F.; Danede, F.; Willart, J.F.; Karrout, Y.; Siepmann, J. PEO hot melt extrudates for controlled drug delivery: Importance of the molecular weight. J. Drug Deliv. Sci. Technol. 2016, 36, 130–140. [Google Scholar] [CrossRef]
- Ma, L.; Deng, L.; Chen, J. Applications of poly(ethylene oxide) in controlled release tablet systems: A review. Drug Dev. Ind. Pharm. 2014, 40, 845–851. [Google Scholar] [CrossRef] [PubMed]













| Formulation | Content % (w/w) | ||
|---|---|---|---|
| PVPVA | PEO | IND | |
| PVPVA-7.5IND | 92.5 | - | 7.5 |
| PVPVA-15IND | 85.0 | - | 15.0 |
| PVPVA-30IND | 70.0 | - | 30.0 |
| PVPVA-10PEO-7.5IND | 82.5 | 10.0 | 7.5 |
| PVPVA-10PEO-15IND | 75.0 | 10.0 | 15.0 |
| PVPVA-10PEO-30IND | 60.0 | 10.0 | 30.0 |
| Variable | Binary SD | Ternary SD |
|---|---|---|
| Plasticisation temperature (°C) | 190 | 170 |
| Injection chamber temperature (°C) | 160 | 160 |
| Shot size (mm) | 15 | 15 |
| Injection pressure (Bar) | 60 | 60 |
| Injection time (s) | 2 | 2 |
| Holding pressure (Bar) | 20 | 20 |
| Holding time (s) | 6 | 6 |
| Mould temperature (°C) | 35 | 35 |
| Formulation | Tablet Weight (mg) | IND (%) |
|---|---|---|
| PVPVA-15IND | 349 ± 8 | 13.7 ± 0.3 |
| PVPVA-30IND | 360 ± 6 | 26.9 ± 0.9 |
| PVPVA-10PEO-7.5IND | 349 ± 2 | 7.20 ± 0.03 |
| PVPVA-10PEO-15IND | 352 ± 2 | 14.9 ± 0.2 |
| PVPVA-10PEO-30IND | 357 ± 7 | 28 ± 1 |
| Formulation | Tg (°C) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|
| PVPVA-15IND | 95.8 ± 0.7 | - | - |
| PVPVA-30IND | 74 ± 1 | - | - |
| PVPVA-10PEO-7.5IND | 99.2 ± 0.5 | 65.9 ± 0.3 | 8.7 ± 0.4 |
| PVPVA-10PEO-15IND | 94.6 ± 0.2 | 65.3 ± 0.3 | 9 ± 1 |
| PVPVA-10PEO-30IND | 76 ± 2 | 62.5 ± 0.6 | 8 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzoli, R.; Hopkins Jnr, M.; Direur, G.; Gately, N.; Lyons, J.G.; Higginbotham, C.L. Micro-Injection Moulding of Poly(vinylpyrrolidone-vinyl acetate) Binary and Ternary Amorphous Solid Dispersions. Pharmaceutics 2019, 11, 240. https://doi.org/10.3390/pharmaceutics11050240
Pezzoli R, Hopkins Jnr M, Direur G, Gately N, Lyons JG, Higginbotham CL. Micro-Injection Moulding of Poly(vinylpyrrolidone-vinyl acetate) Binary and Ternary Amorphous Solid Dispersions. Pharmaceutics. 2019; 11(5):240. https://doi.org/10.3390/pharmaceutics11050240
Chicago/Turabian StylePezzoli, Romina, Michael Hopkins Jnr, Guillaume Direur, Noel Gately, John G. Lyons, and Clement L. Higginbotham. 2019. "Micro-Injection Moulding of Poly(vinylpyrrolidone-vinyl acetate) Binary and Ternary Amorphous Solid Dispersions" Pharmaceutics 11, no. 5: 240. https://doi.org/10.3390/pharmaceutics11050240
APA StylePezzoli, R., Hopkins Jnr, M., Direur, G., Gately, N., Lyons, J. G., & Higginbotham, C. L. (2019). Micro-Injection Moulding of Poly(vinylpyrrolidone-vinyl acetate) Binary and Ternary Amorphous Solid Dispersions. Pharmaceutics, 11(5), 240. https://doi.org/10.3390/pharmaceutics11050240