Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly-l-arginine Conjugated DSPE-PEG (PLD)
2.3. Preparation of Plain and Modified Cationic Proliposomal Powder by Slurry Method
2.4. Physicochemical Properties of Reconstituted Liposomes
2.4.1. Reconstitution of Proliposomal Powder
2.4.2. Size and Zeta Potential
2.4.3. Encapsulation and Loading Efficiency
2.5. Morphology of the Proliposomal Powder and Reconstituted Liposomes by Scanning Electron Microscopy (SEM)
2.6. In Vitro Study Using Cell Cultures
2.6.1. Cell Line
2.6.2. Cellular Toxicity
2.6.3. Cellular Association
Fluorescence Activated Cell Sorting (FACS) Analysis
Confocal Laser Scanning Electron Microscope (CLSM)
2.7. Statistical Analysis
3. Results and Discussions
3.1. Characterization of Synthesized Poly-l-arginine Conjugated DSPE-PEG
3.1.1. 1H NMR
3.1.2. ATR-FTIR
3.2. Proliposomal Powder
3.2.1. Plain-Cationic Proliposomal Powder: Effect of Lipid Amount and Type of Sugar on the Encapsulation Efficiency of Reconstituted Liposomes
3.2.2. Modified-Cationic Proliposomal Powder: Physicochemical Properties of Reconstituted Modified-Liposomes
Particle Size
Zeta Potential
Encapsulation and Loading Efficiency
Morphology of Proliposomal Powder and Reconstituted Liposomes by SEM
3.3. In Vitro Study in Cell Culture
3.3.1. Cellular Toxicity
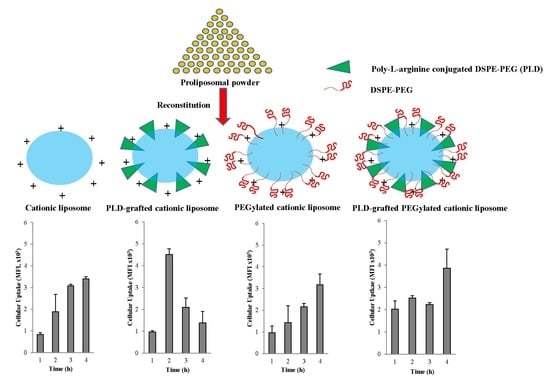
3.3.2. Cellular Association/Uptake According to FACS and CLSM
Fluorescence Activated Cell Sorting (FACS)
CLSM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uda, R.M.; Kato, Y.; Takei, M. Photo-triggered release from liposomes without membrane solubilization, based on binding to poly(vinyl alcohol) carrying a malachite green moiety. Colloids Surf. B Biointerfaces 2016, 146, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Bersani, S.; Vila-Caballer, M.; Brazzale, C.; Barattin, M.; Salmaso, S. PH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine) decorated liposomes for the delivery of gemcitabine to cancer cells. Eur. J. Pharm. Biopharm. 2014, 88, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Guan, Y.; Chang, M.; Zhang, F.; Lu, S.; Wei, T.; Shao, W.; Lin, G. RGD(Arg-Gly-Asp) internalized docetaxel-loaded pH sensitive liposomes: Preparation, characterization and antitumor efficacy in vivo and in vitro. Colloids Surf. B Biointerfaces 2016, 147, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Haeri, A.; Zalba, S.; Hagen, T.L.M.T.; Dadashzadeh, S.; Koning, G.A. EGFR targeted thermosensitive liposomes: A novel multifunctional platform for simultaneous tumor targeted and stimulus responsive drug delivery. Colloids Surf. B Biointerfaces 2016, 146, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiao, Y.; Garg, S.; Song, Y.; Wang, T.; Zhang, N.; Zhang, B. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J. Control Release 2016, 238, 10–21. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Vijaykumar, V.E.; Natarajan, S.K.; Sengupta, S.; Sabbisetti, V.S. Sustained inhibition of cMET-VEGFR2 signaling using liposome-mediated delivery increases efficacy and reduces toxicity in kidney cancer, Nanomedicine Nanotechnology. Biol. Med. 2016, 12, 1853–1861. [Google Scholar] [CrossRef]
- Lee, M.-K. Clinical usefulness of liposomal formulations in cancer therapy: Lessons from the experiences of doxorubicin. J. Pharm. Investig. 2019, 49, 203–214. [Google Scholar] [CrossRef]
- Hao, F.; He, Y.; Sun, Y.; Zheng, B.; Liu, Y.; Wang, X.; Zhang, Y.; Lee, R.J.; Teng, L.; Xie, J. Improvement of oral availability of ginseng fruit saponins by a proliposome delivery system containing sodium deoxycholate. Saudi J. Biol. Sci. 2016, 23, S113–S125. [Google Scholar] [CrossRef]
- Shruthi, M.V.; Parthiban, S.; Senthilkumar, G.P.; Tamizmani, T. Proliposomes as a novel drug delivery system for the improvement of vesicular stability. Int. J. Res. Pharm. Nano Sci. 2014, 3, 326–336. [Google Scholar]
- Akhtar, N.; Ahmad, M.; Ijaz, S.; Ahmad, S.; Sarfraz, M.; Rehman, M.; Al-Kassas, R.; Tahir, N.; Löbenberg, R.; Madni, A. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 2016, 17, 401–426. [Google Scholar] [CrossRef]
- Molnar, D.; Linders, J.; Mayer, C.; Schubert, R. Insertion stability of poly(ethylene glycol)-cholesteryl-based lipid anchors in liposome membranes. Eur. J. Pharm. Biopharm. 2016, 103, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, X.; Shen, B.; Xie, Y.; Shen, C.; Lu, Y.; Qi, J.; Yuan, H.; Wu, W. Enhanced stability of liposomes against solidification stress during freeze-drying and spray-drying by coating with calcium alginate. J. Drug Deliv. Sci. Technol. 2015, 30, 163–170. [Google Scholar] [CrossRef]
- Xiong, P.; He, W.; Cheng, X.; Li, J.; Chen, Y.; Ni, L.; Wei, M. Vitamin E TPGS modified liposomes enhance cellular uptake and targeted delivery of luteolin: An in vivo/in vitro evaluation. Int. J. Pharm. 2016, 512, 262–272. [Google Scholar] [CrossRef]
- Na, K.; Lee, S.A.; Jung, S.H.; Hyun, J.; Shin, B.C. Elastin-like polypeptide modified liposomes for enhancing cellular uptake into tumor cells. Colloids Surf. B Biointerfaces 2012, 91, 130–136. [Google Scholar] [CrossRef]
- Ariën, A.; Goigoux, C.; Baquey, C.; Dupuy, B. Study of in vitro and in vivo stability of liposomes loaded with calcitonin or indium in the gastrointestinal tract. Life Sci. 1993, 53, 1279–1290. [Google Scholar] [CrossRef]
- Daeihamed, M.; Haeri, A.; Ostad, S.N.; Akhlaghi, M.F.; Dadashzadeh, S. Doxorubicin-loaded liposomes: Enhancing the oral bioavailability by modulation of physicochemical characteristics. Nanomedicine 2017, 12, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Tong, S.S.; Xu, Y.; Wang, L.; Fu, M.; Ge, Y.R.; Yu, J.N.; Xu, X.M. Proliposomes for oral delivery of dehydrosilymarin: Preparation and evaluation in vitro and in vivo. Acta Pharmacol. Sin. 2011, 32, 973–980. [Google Scholar] [CrossRef]
- Maniyar, M.G.; Kokare, C.R. Formulation and evaluation of spray dried liposomes of lopinavir for topical application. J. Pharm. Investig. 2019, 49, 259–270. [Google Scholar] [CrossRef]
- Nekkanti, V.; Venkatesan, N.; Betageri, G.V. Proliposomes for oral delivery: Progress and challenges. Curr. Pharm. Biotechnol. 2015, 16, 303–312. [Google Scholar] [CrossRef]
- Witoonsaridsilp, W.; Paeratakul, O.; Panyarachun, B.; Sarisuta, N. Development of mannosylated liposomes using synthesized N-octadecyl-d-mannopyranosylamine to enhance gastrointestinal permeability for protein delivery. AAPS PharmSciTech 2012, 13, 699–706. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Liu, W.; Li, R.; Qin, C.; Liu, N.; Han, J. The preparation of Cistanche phenylethanoid glycosides liquid proliposomes: Optimized formulation, characterization and proliposome dripping pills in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2016, 93, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Nekkanti, V.; Rueda, J.; Wang, Z.; Betageri, G.V. Comparative evaluation of proliposomes and self micro-emulsifying drug delivery system for improved oral bioavailability of nisoldipine. Int. J. Pharm. 2016, 505, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Yousaf, S.; Subramanian, S.; Korale, O.; Alhnan, M.A.; Ahmed, W.; Taylor, K.M.G.; Elhissi, A. Proliposome powders prepared using a slurry method for the generation of beclometasone dipropionate liposomes. Int. J. Pharm. 2015, 496, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Jyoti, K.; Sinha, R.; Katyal, A.; Jain, U.K.; Madan, J. Protamine coated proliposomes of recombinant human insulin encased in Eudragit S100 coated capsule offered improved peptide delivery and permeation across Caco-2 cells. Mater. Sci. Eng. C 2016, 67, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Lim, S.-J.; Lee, M.-K.; Kang, S.N.; Hong, S.-S.; Oh, H. Enhancement of liposomal stability and cellular drug uptake by incorporating tributyrin into celecoxib-loaded liposomes. Asian J. Pharm. Sci. 2013, 8, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Kim, M.-J.; Jeon, S.-O.; Oh, D.-H.; Yoon, K.-H.; Choi, Y.W.; Bashyal, S.; Lee, S. Enhanced topical delivery of fish scale collagen employing negatively surface-modified nanoliposome. J. Pharm. Investig. 2018, 48, 243–250. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Shin, M.C.; Min, K.A.; Huang, Y.; Oh, E.; Moon, C. Cell-penetrating peptide-based non-invasive topical delivery systems. J. Pharm. Investig. 2018, 48, 77–87. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Flynn, N.H. Cell-penetrating peptides transport therapeutics into cells. Pharmacol. Ther. 2015, 154, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafagy, E.S.; Kamei, N.; Takeda-Morishita, M. Cell-penetrating peptide-biodrug strategy for oral and nasal delivery: Review of recent findings. J. Exp. Clin. Med. 2012, 4, 198–202. [Google Scholar] [CrossRef]
- Hossain, M.K.; Cho, H.Y.; Kim, K.J.; Choi, J.W. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, J.M.; Seo, Y.-E.; Saltzman, W.M. Cell penetrating peptide-modified poly(lactic-co-glycolic acid) nanoparticles with enhanced cell internalization. Acta Biomater. 2017, 30, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Åmand, H.L.; Rydberg, H.A.; Fornander, L.H.; Lincoln, P.; Nordén, B.; Esbjörner, E.K. Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2669–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.H.; Kim, C.H.; Yoon, H.Y.; Sung, S.W.; Goh, M.S.; Lee, E.S.; Shin, D.J.; Choi, Y.W. Steric stabilization of RIPL peptide-conjugated liposomes and in vitro assessment. J. Pharm. Investig. 2019, 49, 115–125. [Google Scholar] [CrossRef]
- Kim, H.K.; Davaa, E.; Myung, C.S.; Park, J.S. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int. J. Pharm. 2010, 392, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, N.; Liu, X.J.; Liang, W.; Xu, W.; Torchilin, V.P. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J. Control Release 2006, 112, 229–239. [Google Scholar] [CrossRef]
- Kore, G.; Patil, S.; Misra, A.; Vhora, I.; Baradia, D.; Kolate, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.Y.; Chai, G.H.; Du, Y.Z.; Hu, F.Q. Improved transport and absorption through gastrointestinal tract by pegylated solid lipid nanoparticles. Mol. Pharm. 2013, 10, 1865–1873. [Google Scholar] [CrossRef]
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef]
- Suk, J.S.; Hanes, J.; Ensign, L.M.; Kim, N.; Xu, Q. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2015, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Riedl, A.; Shamsi, Z.; Anderton, M.; Goldfarb, P.; Wiseman, A. Differing features of proteins in membranes may result in antioxidant or prooxidant action: Opposite effects on lipid peroxidation of alcohol dehydrogenase and albumin in liposomal systems. Redox Rep. 1996, 2, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.C.; Lee, S.E.; Kim, T.H.; Ahn, J.B.; Kim, D.H.; Choi, J.S.; Park, J.S. Design of novel proliposome formulation for antioxidant peptide, glutathione with enhanced oral bioavailability and stability. Drug Deliv. 2019, 26, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Kim, M.K.; Paek, H.J.; Choi, S.J.; Oh, J.M. Stable fluorescence conjugation of ZnO nanoparticles and their size dependent cellular uptake. Colloids Surf. B Biointerfaces 2016, 145, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Jung, S.H.; Jung, S.H.; Jeong, K.-S.; Shin, B.C.; Cho, S.H. Polyethylene glycol-complexed cationic liposome for enhanced cellular uptake and anticancer activity. Int. J. Pharm. 2009, 382, 254–261. [Google Scholar] [CrossRef]
- Abdulla, J.M.A.; Tan, Y.T.-F.; Darwis, Y. Rehydrated lyophilized rifampicin-loaded mPEG–DSPE formulations for nebulization. AAPS PharmSciTech 2010, 11, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gu, S.; Ding, Y.; Zhou, L.; Zhang, Z.; Li, L. Electrooxidation and determination of cefotaxime on Au nanoparticles/poly(L-arginine) modified carbon paste electrode. J. Electroanal. Chem. 2013, 698, 25–30. [Google Scholar] [CrossRef]
- Barreira, S.V.P.; Silva, F. Surface modification chemistry based on the electrostatic adsorption of poly-l-arginine onto alkanethiol modified gold surfaces. Langmuir 2003, 19, 10324–10331. [Google Scholar] [CrossRef]
- Nagase, H.; Ueda, H.; Nakagaki, M. Effect of water on lamellar structure of DPPC/sugar systems. Biochim. Biophys. Acta Biomembr. 1997, 1328, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Seki, Y.; Fujihira, S.; Furuta, M.; Deguchi, A.; Goto, M.; Nakata, S.; Nishihara, S.; Fukuhara, K.; Denda, M.; Inoue, K.; et al. Characteristic responses of a phospholipid molecular layer to polyols. Colloids Surf. B Biointerfaces 2015, 136, 594–599. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Narayan, P.; Liu, G.; Panyam, J. Polymer-surfactant nanoparticles for improving oral bioavailability of doxorubicin. J. Pharm. Investig. 2017, 47, 65–73. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int. J. Pharm. 2011, 419, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Song, J.G.; Lee, S.H.; Han, H.-K. The stabilization of biopharmaceuticals: Current understanding and future perspectives. J. Pharm. Investig. 2017, 47, 475–496. [Google Scholar] [CrossRef]
- Xing, L.; Dawei, C.; Liping, X.; Rongqing, Z. Oral colon-specific drug delivery for bee venom peptide: Development of a coated calcium alginate gel beads-entrapped liposome. J. Control Release 2003, 93, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Sriwongsitanont, S.; Ueno, M. Effect of a PEG lipid (DSPE-PEG2000) and freeze-thawing process on phospholipid vesicle size and lamellarity. Colloid Polym. Sci. 2004, 282, 753–760. [Google Scholar] [CrossRef]
- Tirosh, O.; Barenholz, Y.; Katzhendler, J.; Priev, A. Hydration of polyethylene glycol-grafted liposomes. Biophys. J. 1998, 74, 1371–1379. [Google Scholar] [CrossRef]
- Silvander, M.; Johnsson, M.; Edwards, K. Effects of PEG-lipids on permeability of phosphatidylcholine/cholesterol liposomes in buffer and in human serum. Chem. Phys. Lipids 1998, 97, 15–26. [Google Scholar] [CrossRef]
- Meyer, O.; Woodle, M.C.; Park, J.W.; Papahadjopoulos, D.; Kirpotin, D.; Sternberg, B.; Hong, K. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides. J. Biol. Chem. 2002, 273, 15621–15627. [Google Scholar] [CrossRef]
- Dass, C.R. Cytotoxicity issues pertinent to lipoplex-mediated gene therapy in-vivo. J. Pharm. Pharmacol. 2002, 54, 593–601. [Google Scholar] [CrossRef]
- Dass, C.R. Liposome-mediated delivery of oligodeoxynucleotides in vivo. Drug Deliv. J. Deliv. Target Ther. Agents 2002, 9, 169–180. [Google Scholar] [CrossRef]
- Suzuki, T.; Futaki, S.; Niwa, M.; Tanaka, S.; Ueda, K.; Sugiura, Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002, 277, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; Steinman, L.; Kim, D.T.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Console, S.; Marty, C.; García-Echeverría, C.; Schwendener, R.; Ballmer-Hofer, K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J. Biol. Chem. 2003, 278, 35109–35114. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, R.N.; Chan, C.L.; Ewert, K.K.; Silva, B.F.B.; Liang, K.S.; Jacovetty, E.L.; Carragher, B.; Potter, C.S.; Safinya, C.R. Uptake and transfection efficiency of PEGylated cationic liposome-DNA complexes with and without RGD-tagging. Biomaterials 2014, 35, 4996–5005. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.P.; Herlihy, K.P.; DeSimone, J.M.; Luft, J.C.; Reuter, K.G.; Perry, J.L.; Bear, J.E.; Napier, M.; Jones, S.W. PEGylated PRINT Nanoparticles: The impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef]






| Factor | Formulation (Sugar:Protein:Lipid) | Mannitol (gram) | Sucrose (gram) | Protein (gram) | Total Lipid a (gram) |
|---|---|---|---|---|---|
| Type of sugar | 10:0.5:1SUC | - | 10 | 0.5 | 1 |
| 10:0.5:1MAN | 10 | - | 0.5 | 1 | |
| Amount of lipid | 10:0.5:1MAN | 10 | - | 0.5 | 1 |
| 10:0.5:1.5MAN b | 10 | - | 0.5 | 1.5 |
| Modified Substances (MS) | Formulation Code | Compositions (Mole%) DOTAP:DOPE:Chol:(MS) |
|---|---|---|
| None | CATL | 75:20:5: (0) |
| PLD | PLD-CATL | 70:20:5: (5) |
| DSPE-PEG | PEG CATL | 70:20:5: (5) |
| - | 0.5 PEG CATL | 74.5:20:5: (0.5) |
| PLD plus DSPE-PEG | PLD-PEG CATL | 65:20:5: (5 plus 5) |
| Formulations | Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | Zeta Potential (mV) | Encapsulation (%) | Loading (%) | |||
| DI Water | PBS, pH 6.8 | DI Water | PBS, pH 6.8 | ||||
| CATL | 300.6 ± 4.7 | 3633.0 ± 286.3 | 0.40 ± 0.05 | 0.31 ± 0.12 | 44.8 ± 1.6 | 76.2 ± 2.3 | 25.4 ± 0.8 |
| 0.5% PEG CATL | 268.3 ± 13.5 | 2639.7 ± 341.1 | 0.34 ± 0.13 | 0.34 ± 0.13 | 21.9 ± 1.2 | 88.3 ± 0.4 * | 29.4 ± 0.1 * |
| PEG CATL | 212.3 ± 3.7 | 360.3 ± 10.4 | 0.39 ± 0.08 | 0.30 ± 0.06 | −0.2 ± 0.2 | 94.0 ± 0.6 * | 31.0 ± 0.2 * |
| PLD-CATL | 334.9 ± 7.2 | 2650.0 ± 183.9 | 0.46 ± 0.02 | 0.55 ± 0.12 | 44.2 ± 2.9 | 93.3 ± 0.3 * | 31.1 ± 0.1 * |
| PLD-PEG CATL | 206.7 ± 4.7 | 348.3 ± 1.9 | 0.39 ± 0.03 | 0.45 ± 0.01 | −1.1 ± 1.0 | 93.8 ± 0.6 * | 31.3 ± 0.2 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunsirikongkon, A.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Park, J.-S. Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug. Pharmaceutics 2019, 11, 272. https://doi.org/10.3390/pharmaceutics11060272
Tunsirikongkon A, Pyo Y-C, Kim D-H, Lee S-E, Park J-S. Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug. Pharmaceutics. 2019; 11(6):272. https://doi.org/10.3390/pharmaceutics11060272
Chicago/Turabian StyleTunsirikongkon, Amolnat, Yong-Chul Pyo, Dong-Hyun Kim, Sang-Eun Lee, and Jeong-Sook Park. 2019. "Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug" Pharmaceutics 11, no. 6: 272. https://doi.org/10.3390/pharmaceutics11060272
APA StyleTunsirikongkon, A., Pyo, Y. -C., Kim, D. -H., Lee, S. -E., & Park, J. -S. (2019). Optimization of Polyarginine-Conjugated PEG Lipid Grafted Proliposome Formulation for Enhanced Cellular Association of a Protein Drug. Pharmaceutics, 11(6), 272. https://doi.org/10.3390/pharmaceutics11060272