Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Drug Administration, Sampling Procedure, and Linezolid Quantification
2.3. Population Pharmacokinetic Model
2.3.1. Base Model
2.3.2. Covariate Selection
2.3.3. Model Evaluation
2.3.4. External Validation
2.4. PK/PD Analysis in the Study Population
3. Results
3.1. Population Pharmacokinetic Model
3.1.1. Base Model
3.1.2. Covariate Selection
3.1.3. Model Evaluation
3.1.4. External Validation
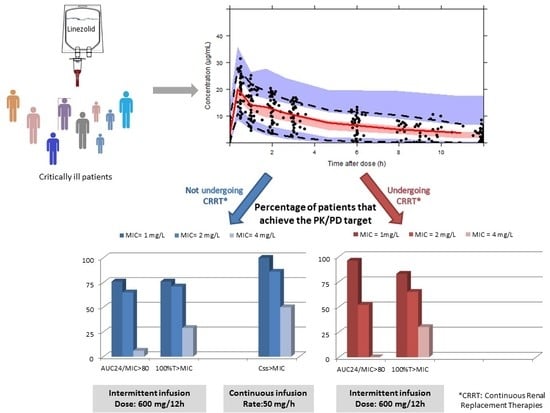
3.2. PK/PD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Savage, R.D.; Fowler, R.A.; Rishu, A.H.; Bagshaw, S.M.; Cook, D.; Dodek, P.; Hall, R.; Kumar, A.; Lamontagne, F.; Lauzier, F.; et al. Pathogens and antimicrobial susceptibility profiles in critically ill patients with bloodstream infections: A descriptive study. CMAJ Open 2016, 4, E569–E577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassetti, M.; Righi, E.; Carnelutti, A. Bloodstream infections in the Intensive Care Unit. Virulence 2016, 7, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Choi, G.Y.; Gomersall, C.D.; Lipman, J. How to optimise antimicrobial prescriptions in the Intensive Care Unit: Principles of individualised dosing using pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 2012, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Ahmed, A.H. Trends in the use of renal replacement therapy modality in intensive care unit: A 7 year study. Ren. Fail. 2015, 37, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Gutiérrez, M.E.; Seller-Pérez, G.; Maynar-Moliner, J.; Sánchez-Izquierdo-Riera, J.A.; Grupo de trabajo “Estado actual del fracaso renal agudo y de las técnicas de reemplazo renal en UCI. Estudio FRAMI”. Epidemiology of acute kidney failure in Spanish ICU. Multi center prospective study FRAMI. Med. Intensiva 2006, 30, 260–267. [Google Scholar] [CrossRef]
- Trotman, R.L.; Williamson, J.C.; Shoemaker, D.M.; Salzer, W.L. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 2005, 41, 1159–1166. [Google Scholar] [CrossRef]
- Roberts, D.M.; Roberts, J.A.; Roberts, M.S.; Liu, X.; Nair, P.; Cole, L.; Lipman, J.; Bellomo, R. RENAL Replacement Therapy Study Investigators. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit. Care Med. 2012, 40, 1523–1528. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Trocóniz, I.F.; Soraluce, A.; Maynar, J.; Sánchez-Izquierdo, J.A.; Isla, A. Population pharmacokinetics of piperacillin and tazobactam in critically ill patients undergoing continuous renal replacement therapy: Application to pharmacokinetic/pharmacodynamic analysis. J. Antimicrob. Chemother. 2014, 69, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Brier, M.E.; Stalker, D.J.; Aronoff, G.R.; Batts, D.H.; Ryan, K.K.; O’Grady, M.; Hopkins, N.K.; Jungbluth, G.L. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob. Agents Chemother. 2003, 47, 2775–2780. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. Bioanalytical Method Validation, Guidance for Industry. US Department of Health and Human Services, Center for Drug Evaluation and Research 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 2 March 2018).
- European Medicines Agency. Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009. Available online: http://www.ema.europa.eu/docs/en_GB/document library/Scientific guideline/2011/08/WC500109686.pdf (accessed on 2 March 2018).
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Roberts, J.A. Individualising therapy to minimize bacterial multidrug resistance. Drugs 2018, 78, 621–641. [Google Scholar] [CrossRef]
- Taubert, M.; Zoller, M.; Maier, B.; Frechen, S.; Scharf, C.; Holdt, L.-M.; Frey, L.; Vogeser, M.; Fuhr, U.; Zander, J. Predictors of inadequate linezolid concentrations after standard dosing in critically ill patients. Antimicrob. Agents Chemother. 2016, 60, 5254–5261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, T.; Takesue, Y.; Ikawa, K.; Morikawa, N.; Ueda, T.; Takahashi, Y.; Nakajima, K.; Takeda, K.; Nishi, S. Population pharmacokinetics/pharmacodynamics of linezolid in sepsis patients with and without continuous renal replacement therapy. Int. J. Antimicrob. Agents 2018, 51, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Slatter, J.G.; Stalker, D.J.; Feenstra, K.L.; Welshman, I.R.; Bruss, J.B.; Sams, J.P.; Johnson, M.G.; Sanders, P.E.; Hauer, M.J.; Fagerness, P.E.; et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab. Dispos. 2001, 29, 1136–1145. [Google Scholar]
- MacGowan, A.P. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J. Antimicrob. Chemother. 2003, 51 (Suppl. 2), 17–25. [Google Scholar] [CrossRef]
- Tsuji, Y.; Holford, N.H.; Kasai, H.; Ogami, C.; Heo, Y.; Higashi, Y.; Mizoguchi, A.; To, H.; Yamamoto, Y. Population pharmacokinetics and pharmacodynamics of linezolid-induced thrombocytopenia in hospitalized patients. Br. J. Clin. Pharmacol. 2017, 83, 1758–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pea, F.; Furlanut, M.; Cojutti, P.G.; Cristini, F.; Zamparini, E.; Franceschi, L.; Viale, P. Therapeutic drug monitoring of linezolid: A retrospective monocentric analysis. Antimicrob. Agents Chemother. 2010, 54, 4605–4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunder, S.; Jayaraman, R.; Mahapatra, H.S.; Sathi, S.; Ramanan, V.; Kanchi, P.; Gupta, A.; Daksh, S.K.; Ram, P. Estimation of renal function in the intensive care unit: The covert concepts brought to light. J. Intensive Care 2014, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Barrasa, H.; Soraluce, A.; Isla, A.; Martín, A.; Maynar, J.; Canut, A.; Sánchez-Izquierdo, J.A.; Rodríguez-Gascón, A. Pharmacokinetics of linezolid in critically ill patients on continuous renal replacement therapy: Influence of residual renal function on PK/PD target attainment. J. Crit. Care 2019, 50, 69–76. [Google Scholar] [CrossRef]
- Villa, G.; Di Maggio, P.; De Gaudio, A.R.; Novelli, A.; Antoniotti, R.; Fiaccadori, E.; Adembri, C. Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: A systematic review. Crit. Care 2016, 20, 374. [Google Scholar] [CrossRef] [Green Version]
- Pea, F.; Viale, P.; Lugano, M.; Pavan, F.; Scudeller, L.; Della Rocca, G.; Furlanut, M. Linezolid disposition after standard dosages in critically ill patients undergoing continuous venovenous hemofiltration: A report of 2 cases. Am. J. Kidney Dis. 2004, 44, 1097–1102. [Google Scholar] [CrossRef]
- Meyer, B.; Kornek, G.V.; Nikfardjam, M.; Karth, G.D.; Heinz, G.; Locker, G.J.; Jaeger, W.; Thalhammer, F. Multiple-dose pharmacokinetics of linezolid during continuous venovenous haemofiltration. J. Antimicrob. Chemother. 2005, 56, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, C.; Muller, L.; Wallis, S.C.; Louart, B.; Saissi, G.; Lipman, J.; Lefrant, J.Y.; Roberts, J.A. Population pharmacokinetics of linezolid in critically ill patients on renal replacement therapy: Comparison of equal doses in continuous venovenous haemofiltration and continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 2016, 71, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Watkins, P.B.; Merz, M.; Avigan, M.I.; Kaplowitz, N.; Regev, A.; Senior, J.R. The clinical liver safety assessment best practices workshop: Rationale, goals, accomplishments and the future. Drug Saf. 2014, 37 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Alraish, R.; Wuensch, T.; Kaffarnik, M. Linezolid in liver failure: Exploring the value of the maximal liver function capacity (LiMAx) test in a pharmacokinetic pilot study. Int. J. Antimicrob. Agents 2017, 50, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Lugano, M.; Baccarani, U.; Pavan, F.; Tavio, M.; Adani, G.L.; Della Rocca, G.; Furlanut, M. Biliary penetration and pharmacodynamic exposure of linezolid in liver transplant patients. J. Antimicrob. Chemother. 2009, 63, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.0. 2018. Available online: http://www.eucast.org (accessed on 16 March 2018).
- Richards, G.A.; Brink, A.J. Therapeutic drug monitoring: Linezolid too? Crit. Care 2014, 18, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuta, S.-I.; Yasui, C.; Aihara, T.; Yoshie, H.; Iida, H.; Beppu, N.; Kurimoto, A.; Yanagi, H.; Mitsunobu, M.; Yamanaka, N.; et al. Chronic liver disease increases the risk of linezolid-related thrombocytopenia in methicillin-resistant Staphylococcus aureus-infected patients after digestive surgery. J. Infect. Chemother. 2011, 17, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Echeverría-Esnal, D.; Retamero, A.; Pardos, S.L.; Grau, S. Severe thrombocytopenia caused by linezolid poisoning in an underweight critically ill patient with renal impairment treated with the recommended doses. Enferm. Infecc. Microbiol. Clín. 2016, 34, 213–214. [Google Scholar] [CrossRef]
- Natsumoto, B.; Yokota, K.; Omata, F.; Furukawa, K. Risk factors for linezolid-associated thrombocytopenia in adult patients. Infection 2014, 42, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, D.; Orlando, G.; Cozzi, V.; Cordier, L.; Baldelli, S.; Merli, S.; Fucile, S.; Gulisano, C.; Rizzardini, G.; Clementi, E. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with Gram-positive infections. Int. J. Antimicrob. Agents 2013, 41, 586–589. [Google Scholar] [CrossRef]



| No CRRT | CRRT | |||
|---|---|---|---|---|
| Patient Characteristic | n | Median (Range) | n | Median (Range) |
| Hospital | ||||
| AUH | 17 | 9 | ||
| DOUH | 0 | 13 | ||
| JUH | 0 | 1 | ||
| Demographic Data | ||||
| Age (years) | 72 (22–85) | 68 (37–79) | ||
| Gender (M/F) | 13/4 | 16/7 | ||
| Body weight (kg) | 71 (60–95) | 74 (55–110) | ||
| Height (m) | 1.70 (1.60–1.85) | 1.69 (1.53–1.85) | ||
| BMI (kg/m2) | 24.5 (20.8–31.3) | 25.9 (21.2–33.1) | ||
| Biochemical Data | ||||
| Clcr (mL/min) | 71.2 (11.0–179.5) | 6.0 (0.0–45.6) | ||
| Creatinine (mg/dL) | 0.80 (0.40–2.10) | 1.15 (0.56–2.60) | ||
| Glucose (mg/dL) | 144 (73–187) | 140 (69–210) | ||
| Hemoglobin (g/dL) | 9.85 (7.00–15.50) | 8.50 (6.70–11.40) | ||
| Hematocrit (%) | 30.5 (20.0–46.0) | 26.4 (18.7–34.7) | ||
| Albumin (g/dL) | 2.8 (1.9–4.0) | 2.2 (1.7–3.6) | ||
| Total proteins (g/dL) | 5.8 (4.2–7.4) | 5.2 (2.7–7.3) | ||
| Bilirubin (mg/dL) | 0.65 (0.20–1.30) | 0.80 (0.12–2.60) | ||
| GPT (U/L) | 25 (6–340) | 50 (5–570) | ||
| GOT (U/L) | 33 (16–330) | 43 (8–675) | ||
| APACHE II | 16 (11–36) | 22 (16–34) | ||
| CLEC (L/h) | 23 | 2.51 (0.79–3.09) | ||
| CVVHDF | 18 | 2.61 (0.79–3.09) | ||
| CVVHD | 5 | 1.06 (1.00–2.73) | ||
| Patient Characteristic | n | Median (Range) |
|---|---|---|
| Demographic Data | ||
| Age (years) | 60 (24–84) | |
| Gender (M/F) | 4/7 * | |
| Body weight (kg) | 80 (70–115) | |
| Height (m) | 1.67 (1.65–1.80) | |
| BMI (kg/m2) | 28.5 (24.2–35.5) | |
| Biochemical Data | ||
| Clcr (mL/min) | 111 (45–240) * | |
| Creatinine (mg/dL) | 0.70 (0.50–1.10) * | |
| Glucose (mg/dL) | 136 (114–217) | |
| Hemoglobin (g/dL) | 10.3 (8.2–11.7) | |
| Albumin (g/dL) | 3.0 (2.5–3.6) * | |
| Total proteins (g/dL) | 5.4 (5.3–7.4) * | |
| Bilirubin (mg/dL) | 0.70 (0.50–2.10) | |
| GPT (U/L) | 37 (18–220) | |
| GOT (U/L) | 25 (16–74) | |
| APACHE II | 16 (6–25) * |
| Parameter | Base Model | Final Model | Bootstrap Median (5th–95th Percentile) |
|---|---|---|---|
| Estimate, RSE (%) | Estimate, RSE (%) | ||
| CL (L/h) b = (CLNR + CLR)+ CLEC | 5.59 (13) + CLEC | ||
| CLNR | 2.62 (18) | 2.65 (2.02–3.65) | |
| CLR = θ × (Clcr/44) | 4.35 (19) | 4.33 (2.99–5.84) | |
| CLEC c = Sc × Qef | |||
| V1 (L) d | 16.1 (20) | 16.2 (14) | 16.6 (11.7–24.4) |
| Q (L/h) | 72.3 (18) | 71.7 (14) | 69.5 (40.4–92.0) |
| V2 (L) | 29.1 (8) | 29.0 (7) | 28.6 (23.0–32.6) |
| IIV_CL (%) | 98.7 (10) | 61.5 (9) | 59.3 (48.9–69.4) |
| IIV_V1 (%) | 66.6 (20) | 65.9 (17) | 62.4 (37.7–91.6) |
| Residual error_additive (mg/L) | 0.260 (24) | 0.266 (24) | 0.267 (0.156–0.464) |
| Residual error_proportional (%) | 0.160 (9) | 0.159 (19) | 0.157 (0.122–0.183) |
| 600 mg/12 h 30 min Infusion | 50 mg/h Continuous Infusion | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC24/MIC ≥ 80 | 100% T > MIC | Css ≥ MIC | ||||||
| MIC (mg/L) | CRRT (n = 23) | No CRRT (n = 17) | p-value a | CRRT (n = 23) | No CRRT (n = 17) | p-value a | n = 11 44 plasma samples | p-value b |
| 1 | 22 (96%) | 13 (76%) | 0.07 | 19 (83%) | 13 (76%) | 0.63 | 44 (100%) | 0.009 |
| 2 | 12 (52%) | 11 (65%) | 0.43 | 15 (65%) | 12 (71%) | 0.72 | 38 (86%) | 0.15 |
| 4 | 0 | 1 (6%) | 0.24 | 7 (30%) | 5 (29%) | 0.94 | 22 (50%) | 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soraluce, A.; Barrasa, H.; Asín-Prieto, E.; Sánchez-Izquierdo, J.Á.; Maynar, J.; Isla, A.; Rodríguez-Gascón, A. Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation. Pharmaceutics 2020, 12, 54. https://doi.org/10.3390/pharmaceutics12010054
Soraluce A, Barrasa H, Asín-Prieto E, Sánchez-Izquierdo JÁ, Maynar J, Isla A, Rodríguez-Gascón A. Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation. Pharmaceutics. 2020; 12(1):54. https://doi.org/10.3390/pharmaceutics12010054
Chicago/Turabian StyleSoraluce, Amaia, Helena Barrasa, Eduardo Asín-Prieto, Jose Ángel Sánchez-Izquierdo, Javier Maynar, Arantxazu Isla, and Alicia Rodríguez-Gascón. 2020. "Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation" Pharmaceutics 12, no. 1: 54. https://doi.org/10.3390/pharmaceutics12010054
APA StyleSoraluce, A., Barrasa, H., Asín-Prieto, E., Sánchez-Izquierdo, J. Á., Maynar, J., Isla, A., & Rodríguez-Gascón, A. (2020). Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation. Pharmaceutics, 12(1), 54. https://doi.org/10.3390/pharmaceutics12010054