Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation
Abstract
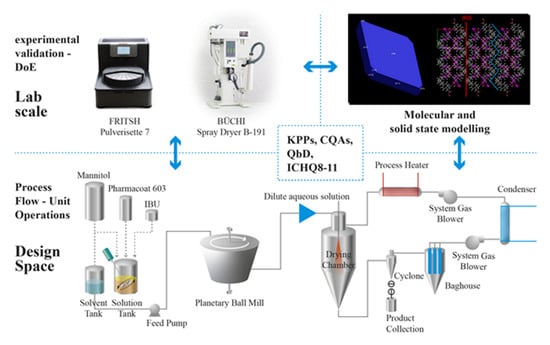
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Wet Media Milling
2.2.2. Selection of Diluent/Co-Milling Agent
2.2.3. Particle Size Determination
2.2.4. Wettability Effect on the Size of Nanocrystals
2.2.5. Solidification of Nanosuspensions by SD
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Determination of Redispersibility
2.2.8. Optimization of WMM by Statistical Design of Experiment (DoE)
2.2.9. Computational Simulation of the Solid State Properties
Molecular and Solid State Modelling
Crystal Morphology Modelling
Lattice Energy Frameworks
Mechanical Properties
Surface Adsorption Simulations
Process Design
3. Results and Discussion
3.1. Wet Media Milling
3.1.1. Stabilizer Selection
3.1.2. Effect of Stabilizer Solution Viscosity and Grinding Beads Diameter
3.1.3. Correlation of Wettability and Surface Energy to the Particle Fracture
3.1.4. Selection of Diluent/Co-Milling Agent
3.2. Optimization of the Wet Media Milling Process
3.2.1. Effects of Drug: Stabilizer Ratio on IBU’s Particle Size
3.2.2. Solidification and Redispersion of IBU Nanosuspension
3.2.3. Statistical Analysis of the Effects of Process Parameters and Formulation Factors on the CQAs of IBU
3.2.4. Design Space Optimization for the Lab Scale Production of IBU Nanocrystals
3.3. Computational Investigation of IBU Fracture Mechanism
3.3.1. Crystal Morphology
3.3.2. Lattice Energy Frameworks
3.3.3. Mechanical Properties
3.3.4. Surface Adsorption Simulations
3.4. Process Design
3.4.1. Modelling of the Wet Media Milling Process
3.4.2. Modelling of the Spray Drying Process
3.4.3. Process Flow Diagram for the Scale-Up of IBU Production for 12 Tons/Year
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Singh, A.; Worku, Z.A.; Van den Mooter, G. Oral formulation strategies to improve solubility of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The Rate Of Solution Of Solid Substances In Their Own Solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, I.; Schenck, D.; Bose, S.; Ruegger, C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: Effect of Vitamin E TPGS and nanocrystal particle size on oral absorption. Eur. J. Pharm. Sci. 2012, 47, 718–728. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Dudognon, E.; Willart, J.F.; Caron, V.; Capet, F.; Larsson, T.; Descamps, M. Formation of budesonide/α-lactose glass solutions by ball-milling. Solid State Commun. 2006, 138, 68–71. [Google Scholar] [CrossRef]
- Branham, M.L.; Moyo, T.; Govender, T. Preparation and solid-state characterization of ball milled saquinavir mesylate for solubility enhancement. Eur. J. Pharm. Biopharm. 2012, 80, 194–202. [Google Scholar] [CrossRef]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamics of stirred media milling. Powder Technol. 2005, 156, 95–102. [Google Scholar] [CrossRef]
- Lu, G.; Duan, Y.-Y.; Wang, X.-D. Surface tension, viscosity, and rheology of water-based nanofluids: A microscopic interpretation on the molecular level. J. Nanopart. Res. 2014, 16, 2564. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Malkin, A.I. Regularities and mechanisms of the Rehbinder’s effect. Colloid J. 2012, 74, 223–238. [Google Scholar] [CrossRef]
- Arpagaus, C. Pharmaceutical Particle Engineering via Nano Spray Drying—Process Parameters and Application Examples on the Laboratory-Scale. Int. J. Med. Nano Res. 2018, 5. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van den Mooter, G. Drying of crystalline drug nanosuspensions-The importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur. J. Pharm. Sci. 2008, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, H. The compressibility and compactibility of powder systems. Int. J. Pharm. 1982, 12, 41–55. [Google Scholar] [CrossRef]
- Larsson, I.; Kristensen, H. Comminution of a brittle/ductile material in a Micros Ring Mill. Powder Technol. 2000, 107, 175–178. [Google Scholar] [CrossRef]
- Yuminoki, K.; Takeda, M.; Kitamura, K.; Numata, S.; Kimura, K.; Takatsuka, T.; Hashimoto, N. Nano-pulverization of poorly water soluble compounds with low melting points by a rotation/revolution pulverizer. Pharmazie 2012, 67, 681–686. [Google Scholar]
- Lestari, M.; Müller, R.; Möschwitzer, J. Systematic Screening of Different Surface Modifiers for the Production of Physically Stable Nanosuspensions. J. Pharm. Sci. 2015, 104. [Google Scholar] [CrossRef]
- Weng, T.; Williams, M. Prevention of Ibuprofen from Forming Low Melting Eutectics with Other Therapeutic Agents in Solid Dosage Forms. U.S. Patent US005512300A, 30 April 1996. [Google Scholar]
- Corvis, Y.; Négrier, P.; Espeau, P. Physicochemical stability of solid dispersions of enantiomeric or racemic ibuprofen in stearic acid. J. Pharm. Sci. 2011, 100, 5235–5243. [Google Scholar] [CrossRef] [Green Version]
- ShinEtsu USP Hypromellose PHARMACOAT Film Coating Material and Binder; Shin Etsu: Wiesbaden, Germany, 2005.
- Nisso HPC Hydroxypropyl Cellulose; Nisso HPC Technical Data Excipients: Niigata, Japan, 2011.
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Yue, P.F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.F.; Wang, C.H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef]
- Fleming, S.; Rohl, A. GDIS: A visualization program for molecular and periodic systems. Z. Krist. 2005, 220, 580–584. [Google Scholar] [CrossRef]
- Gale, J.D.; Rohl, A.L. The General Utility Lattice Program (GULP). Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A Generic Force Field for Molecular Simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Shyjun, S.U.; et al. General Atomic and Molecular Electronic Structure System. Building 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gavezzotti, A. Calculations of lattice energies of organic crystals: The PIXEL integration method in comparison with more traditional methods. Z. Krist. 2005, 220, 499–510. [Google Scholar] [CrossRef]
- Shishkin, O.; Medvediev, V.; Zubatyuk, R. Supramolecular architecture of molecular crystals possessing shearing mechanical properties: Columns versus layers. CrystEngComm 2013, 15, 160–167. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal elastic anisotropy index. Phys. Rev. Lett. 2008, 101, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Gaillac, R.; Pullumbi, P.; Coudert, F.X. ELATE: An open-source online application for analysis and visualization of elastic tensors. J. Phys. Condens. Matter 2016, 28. [Google Scholar] [CrossRef]
- Konkel, J.T.; Myerson, A.S. Empirical molecular modelling of suspension stabilisation with Polysorbate 80. Mol. Simul. 2008, 34, 1353–1357. [Google Scholar] [CrossRef]
- Winn, D.; Doherty, M. Modeling crystal shapes of organic materials grown from solution. AIChE J. 2000, 46, 1348–1367. [Google Scholar] [CrossRef]
- Gavezzotti, A.; Filippini, G.; Kroon, J.; Van Eijck, B.P.; Klewinghaus, P. The crystal polymorphism of tetrolic acid (CH3C≡CCOOH): A molecular dynamics study of precursors in solution, and a crystal structure generation. Chem. Eur. J. 1997, 3, 893–899. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Timko, M.T. Tailoring of specific interactions to modify the morphology of naproxen. J. Cryst. Growth 1999, 205, 233–243. [Google Scholar] [CrossRef]
- Momany, F.; Rone, R. Validation of the General Purpose QUANTA 3.2/CHARMm Force Field. J. Comput. Chem. 1992, 13, 888–900. [Google Scholar] [CrossRef]
- Tuckerman, M.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992, 97, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Peltonen, L. Design space and QbD approach for production of drug nanocrystals by wet media milling techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Lintingre, É.; Ducouret, G.; Lequeux, F.; Olanier, L.; Périé, T.; Talini, L. Controlling the buckling instability of drying droplets of suspensions through colloidal interactions. Soft Matter 2015, 11, 3660–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The influence of mannitol on morphology and disintegration of spray-dried nano-embedded microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef]
- De Souza, J.P. Continuum Representation of the Lennard-Jones Potential for the Electrical Double Layer Structure. Available online: https://web.mit.edu/8.592/www/grades/projects/projects(2018)/PedroDeSouza.pdf (accessed on 15 September 2020).
- Gao, P.; Akrami, A.; Alvarez, F.; Hu, J.; Li, L.; Ma, C.; Surapaneni, S. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J. Pharm. Sci. 2009, 98, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O., III. Enhanced In Vivo Absorption of Itraconazole via Stabilization of Supersaturation Following Acidic-to-Neutral pH Transition. Drug Dev. Ind. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Balani, P.N.; Wong, S.Y.; Ng, W.K.; Widjaja, E.; Tan, R.B.H.; Chan, S.Y. Influence of polymer content on stabilizing milled amorphous salbutamol sulphate. Int. J. Pharm. 2010, 391, 125–136. [Google Scholar] [CrossRef]
- Yani, Y.; Chow, P.; Tan, R. Molecular Simulation Study of the Effect of Various Additives on Salbutamol Sulfate Crystal Habit. Mol. Pharm. 2011, 8, 1910–1918. [Google Scholar] [CrossRef]
- Winn, D.; Doherty, M.F. A new technique for predicting the shape of solution-grown organic crystals. AIChE J. 1998, 44, 2501–2514. [Google Scholar] [CrossRef]
- Cano, H.; Gabas, N.; Canselier, J.P. Experimental study on the ibuprofen crystal growth morphology in solution. J. Cryst. Growth 2001, 224, 335–341. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing agents for drug nanocrystals: Effect on bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kho, H.; Bae, S.; Bae, S.; Kim, B.-W.; Kim, J.S. Planetary Ball Mill Process in Aspect of Milling Energy. J. Korean Powder Metall. Inst. 2014, 21. [Google Scholar] [CrossRef] [Green Version]
- Santhanam, P.R.; Dreizin, E.L. Predicting conditions for scaled-up manufacturing of materials prepared by ball milling. Powder Technol. 2012, 221, 403–411. [Google Scholar] [CrossRef]
- Bond, F.C. Crushing and Grinding Calculations Part I. Br. Chem. Eng. 1961, 6, 378–385. [Google Scholar]
- Couper, J.R.; Penney, W.R.; Fair, J.R.; Walas, S.M. (Eds.) 16—Crystallization from Solutions and Melts. In Chemical Process Equipment, 3rd ed.; Butterworth-Heinemann: Boston, MS, USA, 2012; pp. 561–589. ISBN 978-0-12-396959-0. [Google Scholar]

















| Std | Run | Factor A IBU/HPMC (w/w) | Factor B IBU/Mannitol (w/w) | Factor C Inlet Temperature (°C) |
|---|---|---|---|---|
| 8 | 1 | 5.5 | 2 | 0.88 × Tm |
| 1 | 2 | 2 | 0.5 | 0.88 × Tm |
| 5 | 3 | 5.5 | 1 | 0.88 × Tm |
| 4 | 4 | 2 | 1 | 0.88 × Tm |
| 3 | 5 | 10 | 0.5 | 0.88 × Tm |
| 18 | 6 | 10 | 2 | 1.12 × Tm |
| 7 | 7 | 2 | 2 | 0.88 × Tm |
| 16 | 8 | 2 | 2 | 1.12 × Tm |
| 17 | 9 | 5.5 | 2 | 1.12 × Tm |
| 2 | 10 | 5.5 | 0.5 | 0.88 × Tm |
| 12 | 11 | 10 | 0.5 | 1.12 × Tm |
| 13 | 12 | 2 | 1 | 1.12 × Tm |
| 10 | 13 | 2 | 0.5 | 1.12 × Tm |
| 14 | 14 | 5.5 | 1 | 1.12 × Tm |
| 15 | 15 | 10 | 1 | 1.12 × Tm |
| 9 | 16 | 10 | 2 | 0.88 × Tm |
| 6 | 17 | 10 | 1 | 0.88 × Tm |
| 11 | 18 | 5.5 | 0.5 | 1.12 × Tm |
| Stabilizer | γpsv | γdsv | γsv |
|---|---|---|---|
| Natrosol | 30.14 | 39.19 | 69.33 |
| HPC-SL | 24.30 | 34.22 | 58.52 |
| Pharmacoat 603 | 14.36 | 38.56 | 52.92 |
| HPMC K4M | 12.18 | 44.36 | 56.54 |
| Soluplus | 19.17 | 42.77 | 53.70 |
| Ibuprofen | 36.60 | 24.40 | 51.00 |
| Z-Average (nm)/Run Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 2–13 | 4–12 | 8–7 | 10–18 | 3–14 | 1–9 | 5–11 | 17–15 | 6–16 |
| 3 | 1620 | 3690 | 1910 | 4100 | 6530 | 6970 | 4140 | 6890 | 7120 |
| 6 | 1090 | 1120 | 1330 | 1950 | 3270 | 2500 | 2770 | 2820 | 3630 |
| 9 | 1350 | 712 | 1290 | 1860 | 2700 | 1990 | 3150 | 2230 | 2470 |
| 15 | 1010 | 680 | 1190 | 977 | 2280 | 880 | 2490 | 1570 | 2620 |
| 30 | 755 | 608 | 979 | 1120 | 1200 | 790 | 1620 | 2000 | 2570 |
| 45 | 752 | 438 | 909 | 832 | 714 | 1020 | 2070 | 1680 | 1570 |
| 60 | 462 | 444 | 750 | 648 | 741 | 837 | 1750 | 1320 | 1090 |
| Run Number | Ζ-Potential (mV) |
|---|---|
| 3–14 | −24.5 |
| 5–11 | −34.0 |
| 9–1 | −27.6 |
| 10–18 | −27.5 |
| 17–15 | −27.6 |
| 6–16 | −27.3 |
| 4–12 | −23.2 |
| 7–8 | −24.6 |
| 2–13 | −28.3 |
| Spray Dried at 0.88 Tm (67 °C) | Spray Dried at 1.12 Tm (85 °C) | ||||||
|---|---|---|---|---|---|---|---|
| RUN | Z-Average (nm) | RDI (%) | ζ-potential (mV) | RUN | Z-Average (nm) | RDI (%) | ζ-Potential (mV) |
| 1 | 987 | 117.9 | −11.7 | 9 | 9110 * | 1088 | −11.3 |
| 16 | 1200 | 110.1 | −9.38 | 6 | 1400 | 128.4 | −10.3 |
| 17 | 1360 | 103.0 | −14.3 | 15 | 9950 * | 753.8 | −11.4 |
| 3 | 704 | 95.0 | −12.1 | 14 | 899 | 121.3 | −13.8 |
| 5 | 1960 | 112.0 | −11.6 | 11 | 8860 * | 506.3 | −7.95 |
| 7 | 727 | 96.93 | −12.5 | 8 | 940 | 125.3 | −9.16 |
| 10 | 691 | 106.6 | −16.0 | 18 | 1010 | 155.9 | −12.5 |
| 2 | 575 | 124.5 | −14.4 | 13 | 904 | 195.7 | −12.6 |
| 4 | 642 | 144.6 | −14.9 | 12 | 950 | 214.0 | −13.8 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 6.87 × 107 | 3 | 2.29 × 107 | 3.01 | 0.0656 |
| A | 3.35 × 107 | 1 | 3.35 × 107 | 4.41 | 0.0544 |
| B | 6862 | 1 | 6862 | 0.0009 | 0.9765 |
| C | 3.52 × 107 | 1 | 3.52 × 107 | 4.63 | 0.0493 |
| Residual | 1.06 × 108 | 14 | 7.60 × 106 | ||
| Total | 1.75 × 108 | 17 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 52.09 | 6 | 8.68 | 3.47 | 0.0356 |
| A | 11.13 | 1 | 11.13 | 4.45 | 0.0587 |
| B | 11.30 | 1 | 11.30 | 4.52 | 0.0570 |
| C | 2.76 | 1 | 2.76 | 1.10 | 0.3159 |
| AB | 3.73 | 1 | 3.73 | 1.49 | 0.2479 |
| AC | 2.98 | 1 | 2.98 | 1.19 | 0.2981 |
| BC | 15.75 | 1 | 15.75 | 6.30 | 0.0290 |
| Residual | 27.52 | 11 | 2.50 | - | - |
| Total | 79.61 | 17 | - | - | - |
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | −11.57 | 1 | 0.3766 | −12.40 | −10.74 | |
| A | 0.9689 | 1 | 0.4595 | −0.0424 | 1.98 | 1.02 |
| B | 0.9543 | 1 | 0.4490 | −0.0339 | 1.94 | 1.00 |
| C | 0.3957 | 1 | 0.3766 | −0.4332 | 1.22 | 1.02 |
| AB | −0.6684 | 1 | 0.5477 | −1.87 | 0.5371 | 1.02 |
| AC | 0.4974 | 1 | 0.4554 | −0.5050 | 1.50 | 1.00 |
| BC | −1.12 | 1 | 0.4484 | −2.11 | −0.1381 | 1.02 |
| Mechanical Property | Value |
|---|---|
| Bulk modulus (GPa) | 7.74 |
| Shear modulus (GPa) | 1.66 |
| Compressibility (GPa−1) | 0.13 |
| Young modulus (GPa) | 7.97 |
| Ex | 6.19 |
| Ey | 7.34 |
| Ez | 8.69 |
| Universal anisotropy index | 1.97 |
| Parameter | Unit | Qa | Parameter | Unit | Qa | ||
|---|---|---|---|---|---|---|---|
| Planetary Ball Mill Design Specifications | Ball Milling Media | ||||||
| Disk rotation speed | Ns | rpm | 240 | Diameter | d_b | m | 0.008 |
| Bowl rotation speed | Nb | rpm | 250 | Number | Nbw | - | 5.385 |
| Radius of Sun disk | R | m | 0.3 | Volume | Vb | cm3 | 0.2680 |
| Mill jar diameter | d | m | 0.2118 | Weight | kg | 0.0015 | |
| Mill jar height | h | m | 0.1365 | Weight total | w | kg | 8.199 |
| Mill jar volume | V | cm3 | 4812.38 | IBU mass ratio | - | - | 38.55 |
| Process Parameters of Comminution | |||||||
| Weight of feed | m | kg | 0.2127 | Absolute velocity | Vb | m/s | 6.996 |
| Mechanical efficiency | - | - | 0.8 | Kinetic energy | Kb | joule/hit | 0.037 |
| Specification of Energy | Miscellaneous | ||||||
| Energy per Weight | E | J × h/g | 12,129 | Frequency | f | s−1 | 2.387 |
| Specific Power | - | kJ/kg | 43,666 | Total frequency | ftot | s−1 | 12,855.7 |
| - | - | - | - | Power consumption | P | Watt | 449.53 |
| - | - | - | - | Process time | t | h | 1 |
| Tin | PSD10 | PSD15 | PSD16 | PSD18 | MOIST | TEMP | AIR | SOLID | GAS |
|---|---|---|---|---|---|---|---|---|---|
| MOIST | MOIST | MOIST | |||||||
| °C | 0.0045 mμ | 0.3 mμ | 0.75 mμ | 2.5 mμ | KG/KG DRY | C | KG/HR | KG/HR | KG/KG DRY |
| 84 | 2.43 × 10−6 | 0.016 | 0.051 | 0.230 | 40.08 | 48.5 | 5.21 | 0.000851 | 0.0149 |
| 88 | 2.89 × 10−6 | 0.019 | 0.056 | 0.241 | 34.63 | 49.8 | 5.56 | 0.000851 | 0.0159 |
| 92 | 3.82 × 10−6 | 0.022 | 0.065 | 0.251 | 29.04 | 51.0 | 5.92 | 0.000851 | 0.0170 |
| 96 | 7.21 × 10−6 | 0.029 | 0.079 | 0.255 | 23.31 | 52.2 | 6.28 | 0.000851 | 0.0180 |
| 100 | 8.49 × 10−6 | 0.035 | 0.093 | 0.269 | 17.45 | 53.4 | 6.66 | 0.000851 | 0.0191 |
| 104 | 0.00110 | 0.500 | 0.324 | 0.002 | 2.59 × 10−8 | 50.3 | 7.77 | 1.61 × 10−9 | 0.0223 |
| 108 | 0.00110 | 0.500 | 0.324 | 0.002 | 6.27 × 10−8 | 53.3 | 7.77 | 4 × 10−9 | 0.0223 |
| 112 | 0.00110 | 0.500 | 0.324 | 0.002 | 5.13 × 10−8 | 56.3 | 7.77 | 3.27 × 10−9 | 0.0223 |
| 116 | 0.00110 | 0.500 | 0.324 | 0.002 | 4.79 × 10−8 | 59.3 | 7.77 | 3.06 × 10−9 | 0.0223 |
| 120 | 0.00110 | 0.500 | 0.324 | 0.002 | 3.53 × 10−8 | 62.4 | 7.77 | 2.25 × 10−9 | 0.0223 |
| 124 | 0.00110 | 0.500 | 0.324 | 0.002 | 5.03 × 10−8 | 65.4 | 7.77 | 3.21 × 10−9 | 0.0223 |
| 128 | 0.00110 | 0.500 | 0.324 | 0.002 | 7.36 × 10−8 | 68.4 | 7.77 | 4.7 × 10−9 | 0.0223 |
| 132 | 0.00110 | 0.500 | 0.324 | 0.002 | 5.18 × 10−8 | 71.4 | 7.77 | 3.31 × 10−9 | 0.0223 |
| 136 | 0.00110 | 0.500 | 0.324 | 0.002 | 6.18 × 10−8 | 74.5 | 7.77 | 3.94 × 10−9 | 0.0223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouranidis, A.; Gkampelis, N.; Vardaka, E.; Karagianni, A.; Tsiptsios, D.; Nikolakakis, I.; Kachrimanis, K. Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics 2020, 12, 969. https://doi.org/10.3390/pharmaceutics12100969
Ouranidis A, Gkampelis N, Vardaka E, Karagianni A, Tsiptsios D, Nikolakakis I, Kachrimanis K. Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics. 2020; 12(10):969. https://doi.org/10.3390/pharmaceutics12100969
Chicago/Turabian StyleOuranidis, Andreas, Nikos Gkampelis, Elisavet Vardaka, Anna Karagianni, Dimitrios Tsiptsios, Ioannis Nikolakakis, and Kyriakos Kachrimanis. 2020. "Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation" Pharmaceutics 12, no. 10: 969. https://doi.org/10.3390/pharmaceutics12100969
APA StyleOuranidis, A., Gkampelis, N., Vardaka, E., Karagianni, A., Tsiptsios, D., Nikolakakis, I., & Kachrimanis, K. (2020). Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics, 12(10), 969. https://doi.org/10.3390/pharmaceutics12100969