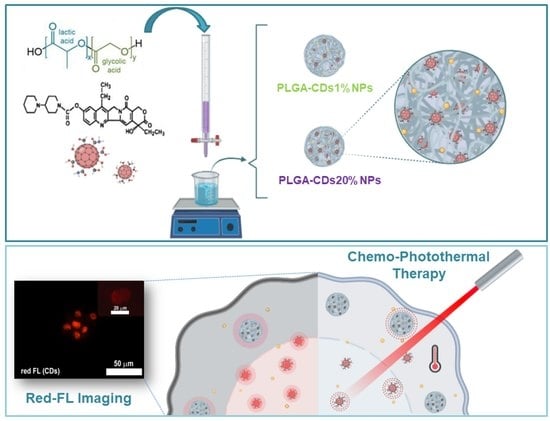
Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Synthesis of CDs
2.4. Size Distribution and Structural Characterization of CDs
2.5. Preparation of PLGA-CDs Nanoparticles by Solvent Displacement Technique (Nanoprecipitation)
2.6. Dynamic Light Scattering Measurements (DLS)
2.7. Atomic Force Microscopy of the PLGA-CDs Nanoparticles (AFM)
2.8. Optical Characterization of the PLGA-CDs Nanoparticles
2.9. Evaluation of Photothermic Effect of PLGA-CDs Nanoparticles
2.10. Drug Loading and Release Kinetics of PLGA-CDs (PLGA-CDs1%@IT and PLGA-CDs20%@IT)
2.11. In Vitro Anticancer Activity of PLGA-CDs20%@IT Nanoparticles
2.12. The 2-D Cell Uptake of PLGA-CDs20%NPs
3. Results and Discussion
3.1. Preparation and Physicochemical Characterization of the Carbon Nanodots
3.2. Preparation of PLGA-CDs Hybrid Nanoparticles and Their Characterization
3.3. Optical and Photothermal Characterization of the PLGA-CDs Nanoparticles
3.4. Preparation of the Irinotecan-loaded PLGA-CDs Nanoparticles
3.5. Evaluation of Irinotecan Loading and Release from PLGA-CDs@IT Nanoparticles
3.6. Biological Characterization of PLGA-CDs20%@IT NPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Kwak, G.; Kim, K.; Kwon, I.C. Theranostic designs of biomaterials for precision medicine in cancer therapy. Biomaterials 2019, 213, 119207. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Rosal, B.; Jia, B.; Jaque, D. Beyond Phototherapy: Recent Advances in Multifunctional Fluorescent Nanoparticles for Light-Triggered Tumor Theranostics. Adv. Funct. Mater. 2018, 28, 1803733. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Cavallaro, G.; Licciardi, M.; Giammona, G. Biotin-containing reduced graphene oxide-based nanosystem as a multieffect anticancer agent: Combining hyperthermia with targeted chemotherapy. Biomacromolecules 2015, 16, 2766–2775. [Google Scholar] [CrossRef]
- Wang, H.; Mukherjee, S.; Yi, J.; Banerjee, P.; Chen, Q.; Zhou, S. Biocompatible Chitosan-Carbon Dot Hybrid Nanogels for NIR-Imaging-Guided Synergistic Photothermal-Chemo Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. [Google Scholar] [CrossRef]
- Geng, B.; Yang, D.; Pan, D.; Wang, L.; Zheng, F.; Shen, W.; Zhang, C.; Li, X. NIR-responsive carbon dots for efficient photothermal cancer therapy at low power densities. Carbon N. Y. 2018, 134, 153–162. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Shen, D.; et al. In vivo theranostics with near-infrared- emitting carbon dots—Highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yan, X.; Kong, D.; Jin, R.; Sun, C.; Du, D. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 2020, 5, 218–234. [Google Scholar] [CrossRef]
- Sciortino, A.; Mauro, N.; Buscarino, G.; Sciortino, L.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Cannas, M.; Messina, F. β-C3N4 Nanocrystals: Carbon Dots with Extraordinary Morphological, Structural, and Optical Homogeneity. Chem. Mater. 2018, 30, 1695–1700. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, S.; Yuan, Z.; Lu, C. Carbon quantum dot-gold nanocluster nanosatellite for ratiometric fluorescence probe and imaging for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 241, 821–827. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, F.; Yang, H.; Ding, C.; Yuan, Z.; Lu, C. Fluorescent sensor array for separation-free dopamine analogue discrimination: Via polyethyleneimine-mediated self-polymerization reaction. Nanoscale 2019, 11, 12889–12897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, L.; Liu, J.; Chen, C.; Zhao, Y. Quantification of Nanomaterial/Nanomedicine Trafficking in Vivo. Anal. Chem. 2018, 90, 589–614. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Li, R.; Wu, W.; Fawcett, J.P.; Gu, J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 97–114. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Ji, J.; Liu, A.; Zhai, G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surf. B Biointerfaces 2016, 148, 460–473. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popoví, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [Green Version]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccines Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Mauro, D.; Cavallaro, G. Near-Infrared, Light-Triggered, On-Demand Anti-inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. J. Carbon Res. 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Craparo, E.F.; Drago, S.E.; Giammona, G.; Cavallaro, G. Production of polymeric micro- and nanostructures with tunable properties as pharmaceutical delivery systems. Polymer 2020, 200, 122596. [Google Scholar] [CrossRef]
- Pitarresi, G.; Martorana, A.; Palumbo, F.S.; Fiorica, C.; Giammona, G. New gellan gum-graft-poly(D,L-lactide-co-glycolide) copolymers as promising bioinks: Synthesis and characterization. Int. J. Biol. Macromol. 2020, 162, 1653–1667. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Scialabba, C.; Sciortino, A.; Messina, F.; Buscarino, G.; Cannas, M.; Roscigno, G.; Condorelli, G.; Cavallaro, G.; Giammona, G.; Mauro, N. Highly Homogeneous Biotinylated Carbon Nanodots: Red-Emitting Nanoheaters as Theranostic Agents toward Precision Cancer Medicine. ACS Appl. Mater. Interfaces 2019, 11, 19854–19866. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Sciortino, A.; Gazzetto, M.; Buscarino, G.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Rohwer, E.J.; Mauro, N.; Feurer, T.; et al. Disentangling size effects and spectral inhomogeneity in carbon nanodots by ultrafast dynamical hole-burning. Nanoscale 2018, 10, 15317–15323. [Google Scholar] [CrossRef]
- Shi, X.; Meng, H.; Sun, Y.; Qu, L.; Lin, Y.; Li, Z.; Du, D. Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in Biotechnology. Small 2019, 15, 1901507. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability Study Perspective of the Effect of Freeze-Drying Using Cryoprotectants on the Structure of Insulin Loaded into PLGA Nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef]
- Hernandez-Giottonini, K.Y.; Rodrıguez-Cordova, R.J.; Gutierrez-Valenzuela, C.A.; Nuri-Miranda, O.P.; ZavalaRivera, P.; Guerrero-German, P.; Lucero-Acuna, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.I.; Shim, Y.H.; Kim, C.; Lim, G.T.; Choi, K.C.; Yoon, C. Effect of cryoprotectants on the reconstitution of surfactant-free nanoparticles of poly(DL-lactide-co-glycolide). J. Microencapsul. 2005, 22, 593–601. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A.; et al. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Scialabba, C.; Agnello, S.; Cavallaro, G.; Giammona, G. Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Mater. Sci. Eng. C 2020, 107, 110201. [Google Scholar] [CrossRef] [PubMed]







| Samples | Pre Lyophilization | Post Lyophilization (with Cryoprotectant) | ||
|---|---|---|---|---|
| Z-Average (d-nm) | PDI | Z-Average (d-nm) | PDI | |
| PLGA-CDs1% | 54.78 | 0.117 | 70 * | 0.224 * |
| PLGA-CDs20% | 74.36 | 0.167 | 93.2 ** | 0.181 ** |
| PLGA-CDs1%@IT | 157.9 | 0.024 | 181.2 * | 0.067 * |
| PLGA-CDs20%@IT | 102.5 | 0.111 | 133.9 ** | 0.120 ** |
| Samples | IC5024 h (μg mL−1) | IC5048 h (μg mL−1) | Imax24 h (%) | Imax48 h (%) |
|---|---|---|---|---|
| Irinotecan | 143.76 | 38 | 51.18 | 83.75 |
| PLGA-CDs20%@IT NPs | 121.12 | 45.10 | 59.80 | 95.79 |
| PLGA-CDs20%@IT NPs + laser | 43.14 | 22.30 | 88.15 | 99.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, N.; Utzeri, M.A.; Drago, S.E.; Buscarino, G.; Cavallaro, G.; Giammona, G. Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics. Pharmaceutics 2020, 12, 1012. https://doi.org/10.3390/pharmaceutics12111012
Mauro N, Utzeri MA, Drago SE, Buscarino G, Cavallaro G, Giammona G. Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics. Pharmaceutics. 2020; 12(11):1012. https://doi.org/10.3390/pharmaceutics12111012
Chicago/Turabian StyleMauro, Nicolò, Mara Andrea Utzeri, Salvatore Emanuele Drago, Gianpiero Buscarino, Gennara Cavallaro, and Gaetano Giammona. 2020. "Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics" Pharmaceutics 12, no. 11: 1012. https://doi.org/10.3390/pharmaceutics12111012
APA StyleMauro, N., Utzeri, M. A., Drago, S. E., Buscarino, G., Cavallaro, G., & Giammona, G. (2020). Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics. Pharmaceutics, 12(11), 1012. https://doi.org/10.3390/pharmaceutics12111012