Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Statistical Analysis
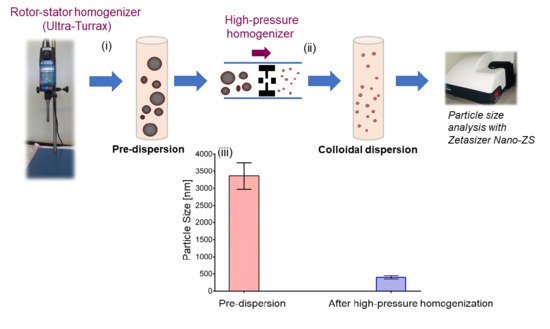
2.3. Preparation of Aqueous Colloidal Dispersions
2.4. Particle Size Analysis
2.5. Density Measurements
2.6. Interfacial Tension and Elasticity
2.7. Confocal Raman Spectral Imaging
3. Results and Discussion
3.1. Confocal Raman Spectral Imaging
3.2. Interfacial Tension and Viscoelasticity
3.3. Particle Size Reduction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehelean, C.A.; Soica, C.M.; Toma, C.-C.; Feflea, S.; Gruia, A.T.; Kasa, P., Jr. Antitumoral activity of betulin, a compound present in birch tree, in formulations with cyclodextrin. Studia Univ. VgSer. St. Vietii 2010, 20, 55–58. [Google Scholar]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a Traditional Medicinal Plant to a Rational Drug: Understanding the Clinically Proven Wound Healing Efficacy of Birch Bark Extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metelmann, H.-R.; Podmelle, F.; Waite, P.D. Long-term cosmetic benefit of wound healing by betuline. Am. J. Cosmet. Surg. 2012, 29, 19–24. [Google Scholar] [CrossRef]
- Steinbrenner, I.; Houdek, P.; Pollok, S.; Brandner, J.M.; Daniels, R. Influence of the Oil Phase and Topical Formulation on the Wound Healing Ability of a Birch Bark Dry Extract. PLoS ONE 2016, 11, e0155582. [Google Scholar] [CrossRef] [Green Version]
- Färber, A.; Daniels, R. Ex vivo Skin Permeation of Betulin from Water-in-Oil Foams. Ski. Pharmacol. Physiol. 2016, 29, 250–256. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, Chemical and Pharmacological Characterization of a New Oleogel-Forming Triterpene Extract from the Outer Bark of Birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract. Pharmaceutics 2020, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [Green Version]
- Pichot, R.; Watson, R.L.; Norton, I.T. Phospholipids at the Interface: Current Trends and Challenges. Int. J. Mol. Sci. 2013, 14, 11767–11794. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwenhuyzen, W. The industrial uses of special lecithins: A review. J. Am. Oil Chem. Soc. 1981, 58, 886–888. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L.; Hartel, R.W. (Eds.) Food Emulsifiers and Their Applications; Springer: New York, NY, USA, 2008; pp. 1–9. [Google Scholar]
- Cabezas, D.M.; Madoery, R.; Diehl, B.W.K.; Tomás, M.C. Emulsifying Properties of Different Modified Sunflower Lecithins. J. Am. Oil Chem. Soc. 2012, 89, 355–361. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Nirschl, H.; Kok, R.J.; Leneweit, G. Adsorption of phospholipids at oil/water interfaces during emulsification is controlled by stress relaxation and diffusion. Soft Matter 2018, 14, 3730–3737. [Google Scholar] [CrossRef]
- Grandell, D.; Murtomäki, L. Surface Pressure Control of Phospholipid Monolayers at the Water/1,2-Dichloroethane Interface. Langmuir 1998, 14, 556–559. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Schmiedel, P. Phase Behavior of Lecithin at the Oil/Water Interface. Langmuir 1996, 12, 6443–6445. [Google Scholar] [CrossRef]
- Langevin, D. Influence of interfacial rheology on foam and emulsion properties. Adv. Colloid Interface Sci. 2000, 88, 209–222. [Google Scholar] [CrossRef]
- Güell, C.; Ferrando, M.; Trentin, A.; Schroën, K. Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems. Membranes 2017, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.G.; Tomás, M.C.; Añón, M.C. Oil-in-water emulsions formulated with sunflower lecithins: Vesicle formation and stability. J. Am. Oil Chem. Soc. 2004, 81, 241–244. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Henry, J.V.L.; Fryer, P.J.; Frith, W.J.; Norton, I.T. The influence of phospholipids and food proteins on the size and stability of model sub-micron emulsions. Food Hydrocoll. 2010, 24, 66–71. [Google Scholar] [CrossRef]
- Broniatowski, M.; Flasiński, M.; Wydro, P. Investigation of the interactions of lupane type pentacyclic triterpenes with outer leaflet membrane phospholipids—Langmuir monolayer and synchrotron X-ray scattering study. J. Colloid Interface Sci. 2012, 381, 116–124. [Google Scholar] [CrossRef]
- Abboud, R.; Charcosset, C.; Greige-Gerges, H. Tetra- and Penta-Cyclic Triterpenes Interaction with Lipid Bilayer Membrane: A Structural Comparative Study. J. Membr. Biol. 2016, 249, 327–338. [Google Scholar] [CrossRef]
- Sznitowska, M.; Janicki, S.; Dabrowska, E.; Zurowska-Pryczkowska, K. Submicron emulsions as drug carriers: Studies on destabilization potential of various drugs. Eur. J. Pharm. Sci. 2001, 12, 175–179. [Google Scholar] [CrossRef]
- Benita, S.; Levy, M.Y. Submicron emulsions as colloidal drug carriers for intravenous administration: Comprehensive physicochemical characterization. J. Pharm. Sci. 1993, 82, 1069–1079. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Chang, C.-C.; Lin, C.-H.; Tsai, Y.-H. Lipid Nano/Submicron Emulsions as Vehicles for Topical Flurbiprofen Delivery. Drug Deliv. 2004, 11, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Klang, S.H.; Baszkin, A.; Benita, S. The stability of piroxicam incorporated in a positively-charged submicron emulsion for ocular administration. Int. J. Pharm. 1996, 132, 33–44. [Google Scholar] [CrossRef]
- Aviv, H.; Friedman, D.; Bar-Ilan, A.; Vered, M. Submicron Emulsions as Ocular Drug Delivery Vehicles. Google Patents US5496811A, 5 March 1996. [Google Scholar]
- Youenang Piemi, M.P.; Korner, D.; Benita, S.; Jean-Paul, M. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J. Control. Release 1999, 58, 177–187. [Google Scholar] [CrossRef]
- Rubinstein, A.; Pathak, Y.V.; Kleinstern, J.; Reches, A.; Benita, S. In Vitro Release and Intestinal Absorption of Physostigmine Salicylate from Submicron Emulsions. J. Pharm. Sci. 1991, 80, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.S.; Weisspapir, M.R.; Friedman, D.I. Enhanced Transdermal Delivery of Diazepam by Submicron Emulsion (SME) Creams. Pharm. Res. 1995, 12, 687–692. [Google Scholar] [CrossRef]
- Sznitowska, M.; Dabrowska, E.A.; Janicki, S. Solubilizing potential of submicron emulsions and aqueous dispersions of lecithin. Int. J. Pharm. 2002, 246, 203–206. [Google Scholar] [CrossRef]
- Cortés-Muñoz, M.; Chevalier-Lucia, D.; Dumay, E. Characteristics of submicron emulsions prepared by ultra-high pressure homogenisation: Effect of chilled or frozen storage. Food Hydrocoll. 2009, 23, 640–654. [Google Scholar] [CrossRef]
- Sjöström, B.; Bergenståhl, B.; Kronberg, B. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. II: Influence of the emulsifier, the solvent, and the drug substance. J. Pharm. Sci. 1993, 82, 584–589. [Google Scholar]
- Rott, C. Herstellung und Charakterisierung Betulinhaltiger Zubereitungen für Berührungsempfindliche Haut. Ph.D. Thesis, University of Tuebingen, Tuebingen, Germany, 2016. [Google Scholar]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of interfacial layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Javadi, A.; Mucic, N.; Karbaschi, M.; Won, J.; Lotfi, M.; Dan, A.; Ulaganathan, V.; Gochev, G.; Makievski, A.; Kovalchuk, V. Characterization methods for liquid interfacial layers. Eur. Phys. J. Spec. Top. 2013, 222, 7–29. [Google Scholar] [CrossRef]
- Miller, R.; Wüstneck, R.; Krägel, J.; Kretzschmar, G. Dilational and shear rheology of adsorption layers at liquid interfaces. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 75–118. [Google Scholar] [CrossRef]
- Heck, R.; Hermann, S.; Lunter, D.J.; Daniels, R. Film-forming formulations containing porous silica for the sustained delivery of actives to the skin. Eur. J. Pharm. Biopharm. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Li, J.B.; Kretzschmar, G.; Miller, R.; Möhwald, H. Viscoelasticity of phospholipid layers at different fluid interfaces. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 491–497. [Google Scholar] [CrossRef]
- Flasiński, M.; Hąc-Wydro, K.; Broniatowski, M. Incorporation of Pentacyclic Triterpenes into Mitochondrial Membrane—Studies on the Interactions in Model 2D Lipid Systems. J. Phys. Chem. B 2014, 118, 12927–12937. [Google Scholar] [CrossRef] [PubMed]
- Sommerling, J.-H.; de Matos, M.B.C.; Hildebrandt, E.; Dessy, A.; Kok, R.J.; Nirschl, H.; Leneweit, G. Instability Mechanisms of Water-in-Oil Nanoemulsions with Phospholipids: Temporal and Morphological Structures. Langmuir 2018, 34, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Opawale, F.O.; Burgess, D.J. Influence of Interfacial Rheological Properties of Mixed Emulsifier Films on the Stability of Water-in-Oil-in-Water Emulsions. J. Pharm. Pharmacol. 1998, 50, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Proust, M. JMP Introductory Guide; SAS Statistical Software: Cary, NC, USA, 2007. [Google Scholar]








| TE Composition | Specific Surface Area | Particle Size D50% |
|---|---|---|
| Betulin 81.60%, lupeol 2.08%, betulinic acid 3.84%, erythrodiol 1.05%, oleanolic acid 0.97%, betulinic acid methyl ester 0.52%, unidentified substances 9.94% | 42 ± 0.4 m2/g | 5.8 µm |
| Batch Code | Pattern | TE (wt %) | PL90H (wt %) | SO (wt %) |
|---|---|---|---|---|
| F1 | 111 | 0.5 | 0.5 | 0 |
| F2 | 112 | 0.5 | 0.5 | 0.3 |
| F3 | 113 | 0.5 | 0.5 | 0.5 |
| F4 | 114 | 0.5 | 0.5 | 1 |
| F5 | 121 | 0.5 | 1 | 0 |
| F6 | 122 | 0.5 | 1 | 0.3 |
| F7 | 123 | 0.5 | 1 | 0.5 |
| F8 | 124 | 0.5 | 1 | 1 |
| F9 | 131 | 0.5 | 2.5 | 0 |
| F10 | 132 | 0.5 | 2.5 | 0.3 |
| F11 | 133 | 0.5 | 2.5 | 0.5 |
| F12 | 134 | 0.5 | 2.5 | 1 |
| Batch Code | Pattern | TE (wt %) | PL90H (wt %) | SO (wt %) | PD (nm) | Particle Size [nm] | Dv (d.nm) | Span | PDI | Predicted Particle Size (nm) | Predicted PDI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 111 | 0.5 | 0.5 | 0 | 2486 ± 379 | 1049 ± 176 | D10: 93 D50: 510 D90: 769 | 1.3 | 0.7 | 1063 | 0.8 |
| F2 | 112 | 0.5 | 0.5 | 0.3 | 1838 ± 405 | 1174 ± 365 | D10: 33 D50: 407 D90: 642 | 1.5 | 0.8 | 1013 | 0.8 |
| F3 | 113 | 0.5 | 0.5 | 0.5 | 1738 ± 220 | 1033 ± 160 | D10: 54 D50: 392 D90: 547 | 1.3 | 0.8 | 981 | 0.8 |
| F4 | 114 | 0.5 | 0.5 | 1 | 1988 ± 343 | 878 ± 292 | D10: 82 D50: 690 D90: 930 | 1.2 | 0.7 | 898 | 0.7 |
| F5 | 121 | 0.5 | 1 | 0 | 1147 ± 167 | 866 ± 215 | D10: 61 D50: 451 D90: 748 | 1.5 | 0.7 | 930 | 0.7 |
| F6 | 122 | 0.5 | 1 | 0.3 | 1968 ± 59 | 935 ± 39 | D10: 85 D50: 554 D90: 788 | 1.3 | 0.7 | 881 | 0.7 |
| F7 | 123 | 0.5 | 1 | 0.5 | 2982 ± 25 | 730 ± 114 | D10: 36 D50: 72 D90: 403 | 5.1 | 0.7 | 848 | 0.7 |
| F8 | 124 | 0.5 | 1 | 1 | 1127 ± 108 | 655 ± 48 | D10: 76 D50: 429 D90: 596 | 1.2 | 0.7 | 766 | 0.7 |
| F9 | 131 | 0.5 | 2.5 | 0 | 1799 ± 165 | 382 ± 35 | D10: 58 D50: 299 D90: 554 | 1.7 | 0.5 | 533 | 0.5 |
| F10 | 132 | 0.5 | 2.5 | 0.3 | 2420 ± 556 | 589 ± 36 | D10: 415 D50: 678 D90: 1070 | 0.9 | 0.4 | 484 | 0.5 |
| F11 | 133 | 0.5 | 2.5 | 0.5 | 1892 ± 253 | 525 ± 48 | D10: 59 D50: 524 D90: 866 | 1.5 | 0.5 | 451 | 0.5 |
| F12 | 134 | 0.5 | 2.5 | 1 | 3400 ± 390 | 400 ± 49 | D10: 57 D50: 152 D90: 400 | 2.3 | 0.5 | 370 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwiiri, F.K.; Daniels, R. Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer. Pharmaceutics 2020, 12, 832. https://doi.org/10.3390/pharmaceutics12090832
Mwiiri FK, Daniels R. Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer. Pharmaceutics. 2020; 12(9):832. https://doi.org/10.3390/pharmaceutics12090832
Chicago/Turabian StyleMwiiri, Francis Kamau, and Rolf Daniels. 2020. "Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer" Pharmaceutics 12, no. 9: 832. https://doi.org/10.3390/pharmaceutics12090832
APA StyleMwiiri, F. K., & Daniels, R. (2020). Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer. Pharmaceutics, 12(9), 832. https://doi.org/10.3390/pharmaceutics12090832