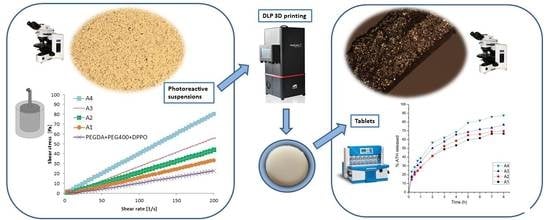
Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Photoreactive Suspensions
2.2.2. Determination of Drug Content in Photopolymer Suspensions
2.2.3. Determination of Drug Content in Tablets
2.2.4. Refractive Index of the Suspension Measurements
2.2.5. Rheological Measurements
2.2.6. 3D Printing of ATH Tablets
2.2.7. Determination of Mass, Dimension and Tensile Strength of Tablets
2.2.8. Polarized Light Microscopy
2.2.9. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.10. In Vitro Drug Release Testing
2.2.11. Kinetic Modeling of Drug Release
3. Results and Discussion
3.1. Drug Content in Photopolymer Suspensions and Tablets
3.2. Refractive Index of The Suspensions Measurements
3.3. Rheological Characterization of Photoreactive Suspensions
3.4. Polarized Light Microscopy of the ATH Powder and Photoreactive Suspensions
3.5. 3D Printing Process
3.6. Appearance, Mass, Doses, and Dimension of the Tablets
3.7. Tensile Strength of the Tablets
3.8. Polarized Light Microscopy of the Tablets Cross-Section
3.9. Fourier Transform Infrared Spectroscopy (FT-IR)
3.10. In Vitro ATH Release
3.11. ATH Release Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: Screening polymers for drug release, drug-polymer miscibility and printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Krkobabić, M.; Medarević, D.; Cvijić, S.; Grujić, B.; Ibrić, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef]
- Katstra, W.E.; Palazzolo, R.D.; Rowe, C.W.; Giritlioglu, B.; Teung, P.; Cima, M.J. Oral dosage forms fabricated by Three Dimensional Printing(TM). J. Control Release 2000, 66, 1–9. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharm. 2020, 577, 119066. [Google Scholar] [CrossRef]
- Madla, C.M.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing technologies, implementation and regulation: An overview. In 3D Printing of Pharmaceuticals; AAPS Advances in the Pharmaceutical Sciences Series; Basit, A.W., Gaisford, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 31, pp. 21–40. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, D. Additive Manufacturing Technologies, 2nd ed.; Springer: New York, NY, USA, 2015; pp. 63–103. [Google Scholar] [CrossRef]
- Schmidt, J.; Colombo, P. Digital light processing of ceramic components from polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly Stretchable and UV Curable Elastomers for Digital Light Processing Based 3D Printing. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Su, P.C.; Wei, J. Curing characteristics of shape memory polymers in 3D projection and laser stereolithography. Virtual Phys. Prototyp. 2017, 12, 77–84. [Google Scholar] [CrossRef]
- Varghese, G.; Moral, M.; Castro-García, M.; López-López, J.J.; Marín-Rueda, J.R.; Yagüe-Alcaraz, V.; Hernández-Afonso, L.; Ruiz-Morales, J.C.; Canales-Vázquez, J. Fabrication and characterisation of ceramics via low-cost DLP 3D printing. Bol. La Soc. Esp. Ceram. Y Vidr. 2018, 57, 9–18. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, Y.S.; Jung, H.D.; Hwang, C.J.; Baik, H.S.; Cha, J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhou, Y.; Lin, X.; Yang, Q.; Yang, G. Printability of external and internal structures based on digital light processing 3D printing technique. Pharmaceutics 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles Martinez, P.; Basit, A.W.; Gaisford, S. The history, developments and opportunities of stereolithography. In 3D Printing of Pharmaceuticals; AAPS Advances in the Pharmaceutical Sciences Series; Basit, A.W., Gaisford, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 31, pp. 55–79. [Google Scholar] [CrossRef]
- Yasmin, M.; Gupta, M.; Shukla, J.P. Molecular interactions and structural effects on mixing pentanol in polyethylene glycol diacrylate and polyethylene glycol dimethacrylate. J. Mol. Liq. 2011, 164, 212–217. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS Pharm. Sci. Tech. 2018, 19, 3355–3361. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, M.; Graule, T.; Hazan, Y.d.; Kata, D.; Lis, J. Highly loaded UV curable nanosilica dispersions for rapid prototyping applications. J. Eur. Ceram. Soc. 2009, 29, 2259–2265. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale, 36th ed.; Pharmaceutical Press: London, UK, 2009; p. 2151. [Google Scholar]
- Bhitre, M.J.; Bhanage, B.; Shirgaonkar, S.J.; Pawar, A.S. Formulation and evaluation of elementary osmotic pump tablet of atomoxetine hydrochloride. Int. J. Pharm. Bio. Sci. 2013, 3, 118–134. [Google Scholar]
- Madzarevic, M.; Medarevic, D.; Vulovic, A.; Sustersic, T.; Djuris, J.; Filipovic, N.; Ibric, S. Optimization and prediction of ibuprofen release from 3D DLP printlets using artificial neural networks. Pharmaceutics 2019, 11, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ChemBK. Available online: https://www.chembk.com/en/chem/Atomoxetine (accessed on 21 August 2020).
- Chartier, T.; Chaput, C.; Doreau, F.; Loiseau, M. Stereolithography of structural complex ceramic parts. J. Mater. Sci. 2002, 37, 3141–3147. [Google Scholar] [CrossRef]
- Mezger, T.G. Applied Rheology, 1st ed.; Anton Paar GmbH: Graz, Austria, 2015. [Google Scholar]
- Konijn, B.J.; Sanderink, O.B.J.; Kruyt, N.P. Experimental study of the viscosity of suspensions: Effect of solid fraction, particle size and suspending liquid. Powder Technol. 2014, 266, 61–69. [Google Scholar] [CrossRef]
- Luo, Y.; Le Fer, G.; Dean, D.; Becker, M.L. 3D printing of poly (propylene fumarate) oligomers: Evaluation of resin viscosity, printing characteristics and mechanical properties. Biomacromolecules 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Sauer, J.; Ring, B.J.; Witcher, J.W. Clinical pharmacokinetics of atomoxetine. Clin. Pharmacokinet. 2005, 44, 571–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, L.; Wang, T.; Li, H.; Huang, R.; Zhang, Z.; Wang, Y.; Quan, D. Taste masking of water-soluble drug by solid lipid microspheres: A child-friendly system established by reversed lipid-based nanoparticle technique. J. Pharm. Pharmacol. 2020, 72, 776–786. [Google Scholar] [CrossRef]
- Nutan, M.T.H.; Reddy, I.K. General principles of suspension. In Pharmaceutical Suspensions: For Formulations Development to Manufacturing; Kulshreshtha, A.K., Singh, O.N., Wall, G.M., Eds.; Springer: New York, NY, USA, 2010; pp. 39–65. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Fernández-García, R.; Prada, M.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Oral fixed-dose combination pharmaceutical products: Industrial manufacturing versus personalized 3D printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Frost, M. Light scattering by small particles. J. Water Supply Res. Technol. AQUA 1998, 47, 87–94. [Google Scholar] [CrossRef]
- Mitteramskogler, G.; Gmeiner, R.; Felzmann, R.; Gruber, S.; Hofstetter, C.; Stampfl, J.; Ebert, J.; Wachter, W.; Laubersheimer, J. Light curing strategies for lithography-based additive manufacturing of customized ceramics. Addit. Manuf. 2014, 1, 110–118. [Google Scholar] [CrossRef]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef]
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Clark, E.A.; Alexander, M.R.; Irvine, D.J.; Roberts, C.J.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Hague, R.J.M.; Tuck, C.J.; Wildman, R.D. 3D printing of tablets using inkjet with UV photoinitiation. Int. J. Pharm. 2017, 529, 523–530. [Google Scholar] [CrossRef]
- Pelras, T.; Glass, S.; Scherzer, T.; Elsner, C.; Schulze, A.; Abel, B. Transparent low molecular weight poly (ethylene glycol) diacrylate-based hydrogels as film media for photoswitchable drugs. Polymers 2017, 9, 693. [Google Scholar] [CrossRef] [Green Version]
- Peter, M.; Tayalia, P. An alternative technique for patterning cells on poly (ethylene glycol) diacrylate hydrogels. RSC Adv. 2016, 6, 40878–40885. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Pasquier, E.; Tomanek, B.; Trudel, S. Synthesis, characterization, and evaluation of PEGylated first-row transition metal ferrite nanoparticles as T2 contrast agents for high-field MRI. RSC Adv. 2017, 7, 38125–38134. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, P.; Harini, K. Design and Optimization of thermo-reversible nasal in situ gel of atomoxetine hydrochloride using taguchi orthogonal array design. Dhaka Univ. J. Pharm. Sci. 2019, 18, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Paloma Ballesteros, M.; Keeble, W.; Barbu, E.; Marie Healy, A.; Lalatsa, A.; Serrano, D.R. Personalised 3D printed medicines: Optimising material properties for successful passive diffusion loading of filaments for fused deposition modelling of solid dosage forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzak, M.S.M.I.; Khan, F.; Khan, M.Z.R.; Fatema, K.; Islam, M.S.; Reza, M.S. Effect of channeling agents on the release profile of theophylline from METHOCEL K4M based matrix tablets. Dhaka Univ. J. Pharm. Sci. 2008, 7, 27–32. [Google Scholar] [CrossRef] [Green Version]









| Substance | ATH (% w/w) | PEGDA (% w/w) | PEG 400 (% w/w) | DPPO (% w/w) | |
|---|---|---|---|---|---|
| Formulation | |||||
| A1 | 10.00 | 66.75 | 22.25 | 1.00 | |
| A2 | 15.00 | 63.00 | 21.00 | 1.00 | |
| A3 | 20.00 | 59.25 | 19.75 | 1.00 | |
| A4 | 25.00 | 55.50 | 18.50 | 1.00 | |
| Mathematical Models | Equations |
|---|---|
| Zero-order | Mt/M∞ = K0∙t |
| First-order | Mt/M∞ = 100 [1 − Exp(−K1∙t)] |
| Higuchi model | Mt/M∞ = KH∙t1/2 |
| Korsmeyer-Peppas model | Mt/M∞ = Kp∙tn |
| Formulation | Drug Content in Photoreactive Suspensions | Drug Content in Tablets |
|---|---|---|
| A1 | 100.29 ± 0.87 | 96.46 ± 3.88 |
| A2 | 95.66 ± 1.88 | 95.62 ± 2.93 |
| A3 | 96.78 ± 0.90 | 96.12 ± 1.80 |
| A4 | 95.58 ± 1.07 | 95.35 ± 0.70 |
| Formulation | Refractive Index |
|---|---|
| PEGDA+PEG 400 + photoinitiator | 1.4572 ± 0.0000 |
| A1 | 1.4661 ± 0.0001 |
| A2 | 1.4675 ± 0.0004 |
| A3 | 1.4685 ± 0.0004 |
| A4 | 1.4690 ± 0.0001 |
| Formulation | Mean ± S.D. (μm) | Min (μm) | Max (μm) |
|---|---|---|---|
| A1 | 22.60 ± 12.57 | 4.36 | 63.65 |
| A2 | 21.53 ± 10.14 | 5.29 | 58.50 |
| A3 | 29.61 ± 12.51 | 8.61 | 71.84 |
| A4 | 34.68 ± 13.27 | 13.61 | 72.93 |
| Parameter | Mass ± S.D. (mg) | Dose ± S.D. (mg) | Diameter ± S.D. (mm) | Thickness ± S.D. (mm) | |
|---|---|---|---|---|---|
| Formulation | |||||
| A1 | 122.08 ± 11.69 | 12.21 ± 1.70 | 7.70 ± 0.17 | 1.94 ± 0.17 | |
| A2 | 130.18 ± 10.00 | 19.53 ± 1.50 | 7.84 ± 0.18 | 2.03 ± 0.13 | |
| A3 | 159.81 ± 11.35 | 31.96 ± 2.27 | 8.06 ± 0.30 | 2.07 ± 0.23 | |
| A4 | 160.29 ± 4.71 | 40.07 ± 1.18 | 8.35 ± 0.13 | 2.16 ± 0.08 | |
| Formulation | Model | R2 Adjusted | n Value |
|---|---|---|---|
| A1 | Korsmeyer-Peppas | 0.9893 | 0.413 |
| A2 | Korsmeyer-Peppas | 0.9854 | 0.437 |
| A3 | Korsmeyer-Peppas | 0.9787 | 0.395 |
| A4 | Korsmeyer-Peppas | 0.9944 | 0.388 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krkobabić, M.; Medarević, D.; Pešić, N.; Vasiljević, D.; Ivković, B.; Ibrić, S. Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics 2020, 12, 833. https://doi.org/10.3390/pharmaceutics12090833
Krkobabić M, Medarević D, Pešić N, Vasiljević D, Ivković B, Ibrić S. Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics. 2020; 12(9):833. https://doi.org/10.3390/pharmaceutics12090833
Chicago/Turabian StyleKrkobabić, Mirjana, Djordje Medarević, Nikola Pešić, Dragana Vasiljević, Branka Ivković, and Svetlana Ibrić. 2020. "Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions" Pharmaceutics 12, no. 9: 833. https://doi.org/10.3390/pharmaceutics12090833
APA StyleKrkobabić, M., Medarević, D., Pešić, N., Vasiljević, D., Ivković, B., & Ibrić, S. (2020). Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics, 12(9), 833. https://doi.org/10.3390/pharmaceutics12090833