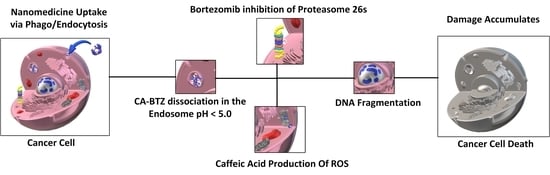
Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanomedicine Synthesis (CAFeB)
2.3. Nanomedicine Characterization
2.4. In Vitro Cell Culture Studies
2.5. Cancer Cytotoxicity Evaluation
2.6. Cell Death Mechanism Study
2.7. In Vitro Dose-Dependent Biocompatibility Study
2.8. Comparison of the Intrinsic Mitochondrial ROS of NIH-3T3 and CT26 Cells
2.9. Image and Statistical Analysis
3. Results
3.1. Chemistry Basis of the Synthesized Nanomedicine
3.2. Characterization of the Supramolecular Prodrug Nanomedicine CAFeB
3.3. In Vitro Dose-Dependent Biocompatibility Analysis
3.4. In Vitro CT26 Cancer Cell Line Antiproliferative Properties of the Nanomedicine
3.5. Cell Death Mechanism Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Cai, X.; Yang, J.; Wang, C.; Tong, L.; Xiao, J.; Li, L. A Targeted and pH-Responsive Bortezomib Nanomedicine in the Treatment of Metastatic Bone Tumors. ACS Appl. Mater. Interfaces 2018, 10, 41003–41011. [Google Scholar] [CrossRef]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907. Available online: https://www.nature.com/articles/nnano.2014.208#supplementary-information (accessed on 18 July 2020). [CrossRef] [PubMed]
- Wang, C.; Sang, H.; Wang, Y.; Zhu, F.; Hu, X.; Wang, X.; Wang, X.; Li, Y.; Cheng, Y. Foe to Friend: Supramolecular Nanomedicines Consisting of Natural Polyphenols and Bortezomib. Nano Lett. 2018, 18, 7045–7051. [Google Scholar] [CrossRef] [PubMed]
- Delatouche, R.; Denis, I.; Grinda, M.; Bahhaj, F.E.; Baucher, E.; Collette, F.; Héroguez, V.; Grégoire, M.; Blanquart, C.; Bertrand, P. Design of pH responsive clickable prodrugs applied to histone deacetylase inhibitors: A new strategy for anticancer therapy. Eur. J. Pharm. Biopharm. 2013, 85, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Chao, C.-y.; Mong, M.-c.; Chan, K.-c.; Yin, M.-c. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2009, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.S.; Na, K. Caffeic acid-coated multifunctional magnetic nanoparticles for the treatment and bimodal imaging of tumours. J. Photochem. Photobiol. B Biol. 2016, 160, 210–216. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Lingström, P.; Örtengren, U.; Niwano, Y. Photo-irradiated caffeic acid exhibits antimicrobial activity against Streptococcus mutans biofilms via hydroxyl radical formation. Sci. Rep. 2017, 7, 6353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Cheng, K.; Zhang, Z.-m.; Fang, R.-q.; Zhu, H.-l. Synthesis, structure and structure–activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamada, K.; Akiyama, J.; Someya, T.; Odaka, Y.; Takeda, Y.; Tori, M.; Nakashima, K.; Maekawa, T.; Sone, Y. Antioxidation mechanism studies of caffeic acid: Identification of antioxidation products of methyl caffeate from lipid oxidation. J. Agric. Food Chem. 2008, 56, 5947–5952. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N. Antibacterial and antioxidant electrospun materials from poly(3-hydroxybutyrate) and polyvinylpyrrolidone containing caffeic acid phenethyl ester—“in” and “on” strategies for enhanced solubility. Int. J. Pharm. 2018, 545, 342–356. [Google Scholar] [CrossRef]
- Aguilar, L.E.; Thomas, R.G.; Moon, M.J.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Implantable chemothermal brachytherapy seeds: A synergistic approach to brachytherapy using polymeric dual drug delivery and hyperthermia for malignant solid tumor ablation. Eur. J. Pharm. Biopharm. 2018, 129, 191–203. [Google Scholar] [CrossRef]
- Aguilar, L.E.; Tumurbaatar, B.; Ghavaminejad, A.; Park, C.H.; Kim, C.S. Functionalized Non-vascular Nitinol Stent via Electropolymerized Polydopamine Thin Film Coating Loaded with Bortezomib Adjunct to Hyperthermia Therapy. Sci. Rep. 2017, 7, 9432. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for pH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195. [Google Scholar] [CrossRef]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Venkata Reddy, B. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Li, X.; Zhen, D.; Lu, X.; Xu, H.e.; Shao, Y.; Xue, Q.; Hu, Y.; Liu, B.; Sun, W. Enhanced cytotoxicity and activation of ROS-dependent c-Jun NH2-terminal kinase and caspase-3 by low doses of tetrandrine-loaded nanoparticles in Lovo cells—A possible Trojan strategy against cancer. Eur. J. Pharm. Biopharm. 2010, 75, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Altayli, E.; Koru, Ö.; Öngörü, Ö.; İde, T.; Açikel, C.; Sarper, M.; Elçi, M.P.; Ilikçi Sağkan, R.; Astarci, E.; Tok, D.; et al. An in vitro and in vivo investigation of the cytotoxic effects of caffeic acid (3,4-dihydroxycinnamic acid) phenethyl ester and bortezomib in multiple myeloma cells. Turk. J. Med. Sci. 2015, 45, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, F.-T. Why bortezomib cannot go with ‘green’? Cancer Biol. Med. 2013, 10, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.D.; Yang, H.J.; Cui, Q.C.; Dou, Q.P.; Chan, T.H. Proteasome inhibition in human breast cancer cells with high catechol-O-methyltransferase activity by green tea polyphenol EGCG analogs. Bioorg. Med. Chem. 2010, 18, 1252–1258. [Google Scholar] [CrossRef] [Green Version]
- Ashley, J.D.; Stefanick, J.F.; Schroeder, V.A.; Suckow, M.A.; Kiziltepe, T.; Bilgicer, B. Liposomal Bortezomib nanoparticles via boronic ester prodrug formulation for improved therapeutic efficacy in vivo. J. Med. Chem. 2014, 57, 5282–5292. [Google Scholar] [CrossRef]
- Zhu, J.H.; Huo, Q.; Xu, M.; Yang, F.; Li, Y.; Shi, H.H.; Niu, Y.M.; Liu, Y. Bortezomib-catechol conjugated prodrug micelles: Combining bone targeting and aryl boronate-based pH-responsive drug release for cancer bone-metastasis therapy. Nanoscale 2018, 10, 18387–18397. [Google Scholar] [CrossRef]
- Gong, N.; Ma, X.; Ye, X.; Zhou, Q.; Chen, X.; Tan, X.; Yao, S.; Huo, S.; Zhang, T.; Chen, S.; et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 2019, 14, 379–387. [Google Scholar] [CrossRef]
- Marques-Fernandez, F.; Planells-Ferrer, L.; Gozzelino, R.; Galenkamp, K.M.; Reix, S.; Llecha-Cano, N.; Lopez-Soriano, J.; Yuste, V.J.; Moubarak, R.S.; Comella, J.X. TNFα induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis. 2013, 4, e493. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Ghosh, S.R.; Weil, A.C.; Zirkin, B.R. Caspase-3 and caspase-activated deoxyribonuclease are associated with testicular germ cell apoptosis resulting from reduced intratesticular testosterone. Endocrinology 2001, 142, 3809–3816. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, L.E.; Jang, S.R.; Park, C.H.; Lee, K.M. Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival. Pharmaceutics 2020, 12, 1082. https://doi.org/10.3390/pharmaceutics12111082
Aguilar LE, Jang SR, Park CH, Lee KM. Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival. Pharmaceutics. 2020; 12(11):1082. https://doi.org/10.3390/pharmaceutics12111082
Chicago/Turabian StyleAguilar, Ludwig Erik, Se Rim Jang, Chan Hee Park, and Kang Min Lee. 2020. "Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival" Pharmaceutics 12, no. 11: 1082. https://doi.org/10.3390/pharmaceutics12111082
APA StyleAguilar, L. E., Jang, S. R., Park, C. H., & Lee, K. M. (2020). Supramolecular Caffeic Acid and Bortezomib Nanomedicine: Prodrug Inducing Reactive Oxygen Species and Inhibiting Cancer Cell Survival. Pharmaceutics, 12(11), 1082. https://doi.org/10.3390/pharmaceutics12111082