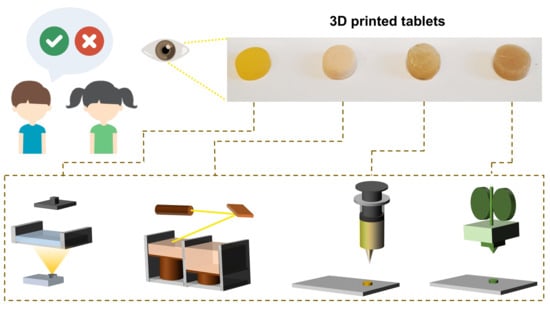
I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the 3D Printed Placebo Printlets
2.2.1. DLP 3D Printing
2.2.2. SLS 3D Printing
2.2.3. Semisolid Extrusion 3D Printing–Chewable Printlets
2.2.4. FDM 3D Printing
2.3. Visual Preference Semi-Structured Survey with Participants
2.4. Statistical Analysis
3. Results
3.1. Printlet Preparation
3.2. Visual Preference of Printlets
3.3. Visual Preference Survey Results Based on Age and Sex
3.4. Visual Printlet Descriptions
4. Discussion
4.1. Visual Preference of Printlets
4.2. Printlet Characteristics for a Quality Target Product Profile (QTPP)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Basit, A.W.; Gaisford, S. 3d Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018; Volume 31. [Google Scholar] [CrossRef]
- Aprecia Pharmaceuticals. Fda Approves the First 3d Printed Drug Product. Available online: https://www.aprecia.com/news/fda-approves-the-first-3d-printed-drug-product (accessed on 8 September 2020).
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3d printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L. Automated therapy preparation of isoleucine formulations using 3d printing for the treatment of msud: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3d printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.; Gaisford, S.; Boyd, B. An overview of 3d printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenfield, S.J.; Xian Tan, H.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to fdm 3d printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3d printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Elbadawi, M.; Castro, B.M.; Gavins, F.K.; Ong, J.J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, Á. M3diseen: A novel machine learning approach for predicting the 3d printability of medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; W Basit, A. 3d printing of tunable zero-order release printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Ong, J.J.; Luzardo-Álvarez, A.; González-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3d printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Gardan, J. Additive manufacturing technologies: State of the art and trends. Int. J. Prod. Res. 2016, 54, 3118–3132. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3d printing for advanced drug delivery and medical device applications. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3d printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective laser sintering 3d printing of orally disintegrating printlets containing ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3d printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3d printed pellets (miniprintlets): A novel, multi-drug, controlled release platform technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (sls) 3d printing of medicines. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [Green Version]
- Preis, M.; Öblom, H. 3d-printed drugs for children—Are we ready yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Strickley, R.G.; Iwata, Q.; Wu, S.; Dahl, T.C. Pediatric drugs—A review of commercially available oral formulations. J. Pharm. Sci. 2008, 97, 1731–1774. [Google Scholar] [CrossRef]
- Rautamo, M.; Kvarnstrom, K.; Siven, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. A focus group study about oral drug administration practices at hospital wards-aspects to consider in drug development of age-appropriate formulations for children. Pharmaceutics 2020, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3d printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Paediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3d printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef] [PubMed]
- Trofimiuk, M.; Wasilewska, K.; Winnicka, K. How to modify drug release in paediatric dosage forms? Novel technologies and modern approaches with regard to children’s population. Int. J. Mol. Sci 2019, 20, 3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3d printed “starmix” drug loaded dosage forms for paediatric applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3d printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [Green Version]
- Pushparajah, D.S. Making patient engagement a reality. Patient 2018, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rautamo, M.; Kvarnstrom, K.; Siven, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and prerequisites associated with the adoption of oral 3d-printed medicines for pediatric patients: A focus group study among healthcare professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef] [Green Version]
- Agency, E.M. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: London, UK, 2013. [Google Scholar]
- El-Rachidi, S.; LaRochelle, J.M.; Morgan, J.A. Pharmacists and pediatric medication adherence: Bridging the gap. Hosp. Pharm. 2017, 52, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the children’s acceptability of oral formulations (calf) study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Fastø, M.M.; Genina, N.; Kaae, S.; Kälvemark Sporrong, S. Perceptions, preferences and acceptability of patient designed 3d printed medicine by polypharmacy patients: A pilot study. Int. J. Clin. Pharm. 2019, 41, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3d printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (sla) 3d printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of geometry on the drug release profiles of stereolithographic (sla) 3d-printed tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (sla) 3d printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 119428. [Google Scholar] [CrossRef]
- Larush, L.; Kaner, I.; Fluksman, A.; Tamsut, A.; Pawar, A.A.; Lesnovski, P.; Benny, O.; Magdassi, S. 3d printing of responsive hydrogels for drug-delivery systems. J. 3D Print. Med. 2017, 1, 219–229. [Google Scholar] [CrossRef]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (sla) 3d printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3d printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3d scanning and 3d printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Xu, X.; Muras, A.; Otero, A.; Concheiro, A.; Gaisford, S.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Anti-biofilm multi drug-loaded 3d printed hearing aids. Mater. Sci. Eng. C 2020, 119, 111606. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Formlabs. High-Accuracy 3d Printing Materials for Dental Labs and Practices. Available online: https://dental.formlabs.com/uk/materials/ (accessed on 18 June 2020).
- Next Dent. Leading Dental Materials for 3d Printing. Available online: https://nextdent.com/ (accessed on 18 June 2020).
- Formlabs. Are Formlabs Resins Food-Safe or Biocompatible? Available online: https://support.formlabs.com/s/article/Are-Formlabs-resins-food-safe-or-biocompatible?language=en_US (accessed on 18 June 2020).
- Alyami, H.; Dahmash, E.; Alyami, F.; Dahmash, D.; Huynh, C.; Terry, D.; Mohammed, A.R. Dosage form preference consultation study in children and young adults: Paving the way for patient-centred and patient-informed dosage form development. Eur. J. Hosp. Pharm. 2017, 24, 332–337. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3d printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.T.; Lv, Z.F.; Tian, P.; Lin, M.M.; Lin, W.; Huang, S.Y.; Chen, Y.Z. Semi-solid extrusion 3d printing odfs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 1–8. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3d printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3d extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H.; SPaeDD-UK Project. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [Green Version]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3d-printed isoniazid tablets for the treatment and prevention of tuberculosis—personalized dosing and drug release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar] [CrossRef] [Green Version]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (pva). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, J.; Wang, M.; Wang, L.; Yang, J. 3d printed polyvinyl alcohol tablets with multiple release profiles. Sci. Rep. 2019, 9, 12487. [Google Scholar] [CrossRef] [PubMed]







| Feature | DLP | SLS | SSE | FDM |
|---|---|---|---|---|
| Age-appropriate formulation design (different sizes and shapes) |  |  |  |  |
| Manufacture of multiparticulate systems |  |  |  |  |
| Manufacture of chewable formulations |  |  |  |  |
| Manufacture of dispersible formulations |  |  |  |  |
| Use of pharmaceutical grade excipients with an established safety profile in the target paediatric age group(s) |  |  |  |  |
| Taste masking capabilities through the addition of sweeteners/flavourings |  |  |  |  |
| Selection of colour |  |  |  |  |
| Aesthetically smooth surface |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. https://doi.org/10.3390/pharmaceutics12111100
Januskaite P, Xu X, Ranmal SR, Gaisford S, Basit AW, Tuleu C, Goyanes A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics. 2020; 12(11):1100. https://doi.org/10.3390/pharmaceutics12111100
Chicago/Turabian StyleJanuskaite, Patricija, Xiaoyan Xu, Sejal R. Ranmal, Simon Gaisford, Abdul W. Basit, Catherine Tuleu, and Alvaro Goyanes. 2020. "I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets" Pharmaceutics 12, no. 11: 1100. https://doi.org/10.3390/pharmaceutics12111100
APA StyleJanuskaite, P., Xu, X., Ranmal, S. R., Gaisford, S., Basit, A. W., Tuleu, C., & Goyanes, A. (2020). I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics, 12(11), 1100. https://doi.org/10.3390/pharmaceutics12111100