Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations
Abstract
:1. Introduction
2. Classification of Biosurfactants
3. Biosurfactants as Promising Alternatives to Chemical Surfactants
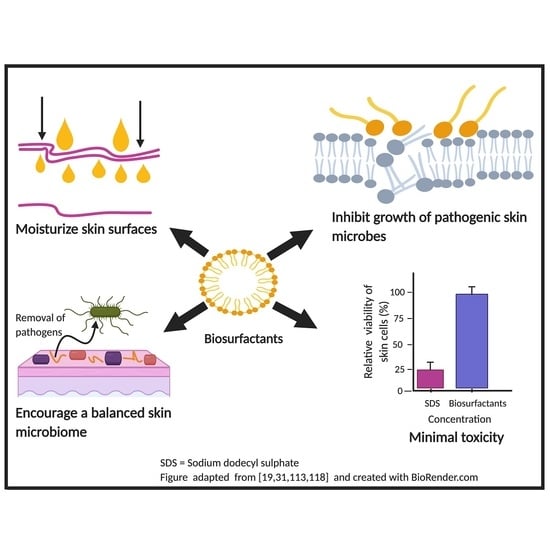
4. Biosurfactants, Human Skin and Its Microbiome
5. Antimicrobial Efficacy of Microbial Biosurfactants
6. Biosurfactants as Skin Surface Moisturizer
7. Cytotoxicity Studies
8. Effects of Biosurfactants on Skin Cell Types
9. Challenges and Solutions for Biosurfactant Applications
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Agarwal, S.; Krishnamurthy, K. Histology, Skin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Simmering, R.; Breves, R. Pre- and probiotic cosmetics. Hautarzt 2009, 60, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Bay, L.; Barnes, C.J.; Fritz, B.G.; Thorsen, J.; Restrup, M.E.M.; Rasmussen, L.; Sørensen, J.K.; Hesselvig, A.B.; Odgaard, A.; Hansen, A.J.; et al. Universal dermal microbiome in human skin. MBio 2020, 11, e02945-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, C.M.; Loomis, K.; Stone, W.; Mehoke, T.; Brensinger, B.; Pellicore, M.; Staniczenko, P.P.A.; Charles, C.; Nayak, S.; Karig, D.K. Isolation and characterization of diverse microbial representatives from the human skin microbiome. Microbiome 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, K.; Heinrich, U.; Tronnier, H. Influence of different cosmetic formulations on the human skin barrier. Skin Pharmacol. Physiol. 2014, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rodan, K.; Fields, K.; Majewski, G.; Falla, T. Skincare Bootcamp. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1152. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of microbial products in cosmetic industry. Nat. Prod. Bioprospect. 2019, 9, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Holland, K.T.; Bojar, R.A. Cosmetics What is Their Influence on the Skin Microflora? J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.E.; Lee, S.; Kim, S.; Han, H.; Jeon, C.O. Effects of cosmetics on the skin microbiome of facial cheeks with different hydration levels. Microbiologyopen 2018, 7, e00557. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; De Rienzo, M.A.D.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, A. Interactions between surfactants and the skin – Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [Green Version]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Sahnoun, R.; Mnif, I.; Fetoui, H.; Gdoura, R.; Chaabouni, K.; Makni-Ayadi, F.; Kallel, C.; Ellouze-Chaabouni, S.; Ghribi, D. Evaluation of Bacillus subtilis SPB1 lipopeptide biosurfactant toxicity towards mice. Int. J. Pept. Res. Ther. 2014, 20, 333–340. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandeep, L. Biosurfactant: Pharmaceutical Perspective. J. Anal. Pharm. Res. 2017, 4. [Google Scholar] [CrossRef]
- Sil, J.; Dandapat, P.; Das, S. Health Care Applications of Different Biosurfactants: Review. Int. J. Sci. Res. 2015, 6, 41–50. [Google Scholar] [CrossRef]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine biosurfactants: Biosynthesis, structural diversity and biotechnological applications. Mar. Drugs 2019, 17, 408. [Google Scholar] [CrossRef] [Green Version]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Peyrat, L.; Tsafantakis, N.; Georgousaki, K.; Ouazzani, J.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Terrestrial Microorganisms: Cell Factories of Bioactive Molecules with Skin Protecting Applications. Molecules 2019, 24, 1836. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Ahmed, S.; Eswari, J.S. Therapeutic cyclic lipopeptides mining from microbes: Latest strides and hurdles. World J. Microbiol. Biotechnol. 2015, 31, 1177–1193. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Lipopeptides in cosmetics. Int. J. Cosmet. Sci. 2010, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.; Sharma, A.; Kanwar, S. Lipopeptides: A Distinct Class of Antibiotics with Diverse Applications. Adv. Biotechnol. Microbiol. 2017, 7, 555706. [Google Scholar] [CrossRef]
- Corazza, M.; Lauriola, M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, skin cleansing protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.-N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef]
- Yanase, K.; Hatta, I. Disruption of human stratum corneum lipid structure by sodium dodecyl sulphate. Int. J. Cosmet. Sci. 2018, 40, 44–49. [Google Scholar] [CrossRef]
- Eckhart, L.; Tschachler, E. Control of cell death-associated danger signals during cornification prevents autoinflammation of the skin. Exp. Dermatol. 2018, 27, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Dykes, P. Surfactants and the skin. Int. J. Cosmet. Sci. 1998, 20, 53–61. [Google Scholar] [CrossRef]
- Moore, D.J.; Rawlings, A.V. The chemistry, function and (patho)physiology of stratum corneum barrier ceramides. Int. J. Cosmet. Sci. 2017, 39, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Spada, F.; Barnes, T.M.; Greive, K.A. Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clin. Cosmet. Investig. Dermatol. 2018, 11, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T. Critical Micelle Concentration. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 209–210. [Google Scholar]
- Morris, S.A.V.; Thompson, R.T.; Glenn, R.W.; Ananthapadmanabhan, K.P.; Kasting, G.B. Mechanisms of anionic surfactant penetration into human skin: Investigating monomer, micelle and submicellar aggregate penetration theories. Int. J. Cosmet. Sci. 2019, 41, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.J.; Weyrich, L.S.; Dixit, S.; Farrer, A.G. The skin microbiome: Associations between altered microbial communities and disease. Australas. J. Dermatol. 2015, 56, 268–274. [Google Scholar] [CrossRef]
- Cundell, A.M. Microbial Ecology of the Human Skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Lunjani, N.; Hlela, C.; O’Mahony, L. Microbiome and skin biology. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 328–333. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [Green Version]
- Cosseau, C.; Romano-Bertrand, S.; Duplan, H.; Lucas, O.; Ingrassia, I.; Pigasse, C.; Roques, C.; Jumas-Bilak, E. Proteobacteria from the human skin microbiota: Species-level diversity and hypotheses. ONEHLT 2016, 2, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Musthaq, S.; Mazuy, A.; Jakus, J. The microbiome in dermatology. Clin. Dermatol. 2018, 36, 390–398. [Google Scholar] [CrossRef]
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermatol. Res. 2018, 310, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, C.; Lucas, R.; Barba, C.; Marti, M.; Rubio, L.; Comelles, F.; Morales, J.C.; Coderch, L.; Parra, J.L. Skin delivery of antioxidant surfactants based on gallic acid and hydroxytyrosol. J. Pharm. Pharmacol. 2015, 67, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Borah, S.N.; Kandimalla, R.; Bora, A.; Deka, S. Sophorolipid Biosurfactant Can Control Cutaneous Dermatophytosis Caused by Trichophyton mentagrophytes. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharaei-Fathabad, E. Biosurfactants in pharmaceutical industry: A mini-review. Am. J. Drug Discov. Dev. 2011, 1, 58–69. [Google Scholar] [CrossRef] [Green Version]
- BASF’s care creations. Available online: https://www.carecreations.basf.com/product-formulations/products/products-detail/RELIPIDIUMBC10096/307139450 (accessed on 28 June 2020).
- SopholianceTM S. Available online: https://www.givaudan.com/fragrances/active-beauty/products/sopholiance-s (accessed on 28 July 2020).
- Findley, K.; Grice, E.A. The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease. PLoS Pathog. 2014, 10, 10–12. [Google Scholar] [CrossRef]
- Yang, Y.; Ashworth, A.J.; Willett, C.; Cook, K.; Upadhyay, A.; Owens, P.R.; Ricke, S.C.; DeBruyn, J.M.; Moore, P.A. Review of Antibiotic Resistance, Ecology, Dissemination, and Mitigation in U.S. Broiler Poultry Systems. Front. Microbiol. 2019, 10, 2639. [Google Scholar] [CrossRef]
- Butler, M.S.; Buss, A.D. Natural products—The future scaffolds for novel antibiotics? Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef]
- Lim, J.S.; Park, H.S.; Cho, S.; Yoon, H.S. Antibiotic susceptibility and treatment response in bacterial skin infection. Ann. Dermatol. 2018, 30, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E. Skin microbiota’s community effort. Nature 2018, 563, S91–S93. [Google Scholar] [CrossRef]
- Dempster, C.; Banat, I.; Mrrchant, R. Antimicrobial and antibiofilm potential of biosurfactants as novel combination therapy against bacterium that cause skin infections. Access Microbiol. 2019, 1, 874. [Google Scholar] [CrossRef]
- Oon, H.H.; Wong, S.N.; Wee, D.C.A.W.; Cheong, W.K.; Goh, C.L.; Tan, H.H. Acne management guidelines by the Dermatological society of Singapore. J. Clin. Aesthet. Dermatol. 2019, 12, 34–50. [Google Scholar] [PubMed]
- Gudiña, E.; Teixeira, J.; Rodrigues, L. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juma, A.; Lemoine, P.; Simpson, A.B.J.; Murray, J.; O’Hagan, B.M.G.; Naughton, P.J.; Dooley, J.G.; Banat, I.M. Microscopic Investigation of the Combined Use of Antibiotics and Biosurfactants on Methicillin Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Anestopoulos, I.; Kiousi, D.E.; Klavaris, A.; Galanis, A.; Salek, K.; Euston, S.R.; Pappa, A.; Panayiotidis, M.I. Surface active agents and their health-promoting properties: Molecules of multifunctional significance. Pharmaceutics 2020, 12, 688. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Ma, F.; Han, S.; Zhang, Y. Oxygen effects on rhamnolipids production by Pseudomonas aeruginosa. Microb. Cell Fact. 2018, 17, 39. [Google Scholar] [CrossRef]
- Saika, A.; Koike, H.; Fukuoka, T.; Morita, T. Tailor-made mannosylerythritol lipids: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6877–6884. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S.; Chauhan, N.; Bansal, A.; Procha, S. Production and Structural Characterization of Lactobacillus helveticus Derived Biosurfactant. Hindawi Publ. Corp. 2014, 2014, 493548. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banpurkar, A.G.; Banat, I.M. Lactobacillus acidophilus derived biosurfactant as a biofilm inhibitor: A promising investigation using microfluidic approach. Appl. Sci. 2018, 8, 1555. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.A.V.; De Arruda, I.R.; Dos Santos, A.F.A.B.; De Araújo, A.A.; Maior, A.M.S.; Ximenes, E.A. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz. J. Microbiol. 2007, 38, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, E.Z. Antimicrobial activity of a biosurfactant produced by bacillus licheniformis strain m104 grown on whey. Braz. Arch. Biol. Technol. 2013, 56, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Urzedo, C.A.; Freitas, Q.; Akemi, V.; Silveira, I.; Pedrine, M.A.; Celligoi, C. Adv Biotech & Micro Antimicrobial Applications of Sophorolipid from Candida bombicola: A Promising Alternative to Conventional Drugs. Adv. Biotechnol. Microbiol. 2018, 9, 555753. [Google Scholar] [CrossRef]
- Nashida, J.; Nishi, N.; Takahashi, Y.; Hayashi, C.; Igarashi, M.; Takahashi, D.; Toshima, K. Systematic and Stereoselective Total Synthesis of Mannosylerythritol Lipids and Evaluation of Their Antibacterial Activity. J. Org. Chem. 2018, 83, 7281–7289. [Google Scholar] [CrossRef]
- Borsanyiova, M.; Patil, A.; Mukherji, R.; Prabhune, A.; Bopegamage, S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. (Praha) 2016, 61, 85–89. [Google Scholar] [CrossRef]
- Bharali, P.; Das, S.; Ray, A.; Pradeep Singh, S.; Bora, U.; Kumar Konwar, B.; Singh, C.B.; Sahoo, D. Biocompatibility natural effect of rhamnolipids in bioremediation process on different biological systems at the site of contamination. Bioremediat. J. 2018, 22, 91–102. [Google Scholar] [CrossRef]
- De Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Sekhon Randhawa, K.K.; Rahman, P.K.S.M. Rhamnolipid biosurfactants- past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef] [Green Version]
- Nian Tan, Y.; Li, Q. Microbial production of rhamnolipids using sugars as carbon sources. Microb. Cell Fact. 2018, 17, 89. [Google Scholar] [CrossRef]
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-biofilm properties of bacterial di-rhamnolipids and their semi-synthetic amide derivatives. Front. Microbiol. 2017, 8, 2454. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, K.V.; Safar, L.; Devi, K. Biofilm in dermatology. J. Ski. Sex. Transm. Dis. 2019, 1, 3–7. [Google Scholar] [CrossRef]
- Sonesson, A.; Przybyszewska, K.; Eriksson, S.; Mörgelin, M.; Kjellström, S.; Davies, J.; Potempa, J.; Schmidtchen, A. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci. Rep. 2017, 7, 8689. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “House of Biofilm Cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial biofilms and the human skin microbiome. NPJ Biofilms Microbiomes 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ. Sci. 2020, 32, 223–227. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Lee, J.H. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Ennis, C.J.; Rahman, P.K.S.M. Production of biosurfactants using eco-friendly microorganisms. In Environmental Sustainability: Role of Green Technologies; Springer: New Delhi, India, 2015; pp. 185–204. [Google Scholar]
- Sharma, D.; Saharan, B.S.; Kapil, S. Biosurfactants of Probiotic Lactic Acid Bacteria, 1st ed.; SpringerBriefs in Microbiology; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–26. [Google Scholar]
- Ghasemi, A.; Moosavi-Nasab, M.; Setoodeh, P.; Mesbahi, G.; Yousefi, G. Biosurfactant Production by Lactic Acid Bacterium Pediococcus dextrinicus SHU1593 Grown on Different Carbon Sources: Strain Screening Followed by Product Characterization. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Huang, W.Y.; Toh, H.S.; Weng, T.C.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial activity of lactobacillus species against carbapenem-resistant enterobacteriaceae. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Kheyrolahzadeh, K.; Mahdi, S.; Khatibi, H.; Sharifi, S.; Memar, M.Y.; Vahed, S.Z. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Singh Saharan, B.; Chauhan, N.; Procha, S.; Lal, S. Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springerplus 2015, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fariq, A.; Saeed, A. Production and Biomedical Applications of Probiotic Biosurfactants. Curr. Microbiol. 2016, 72, 489–495. [Google Scholar] [CrossRef]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P 6A and Lactobacillus gasseri P 65. Microb. Cell Fact. 2017, 16, 155. [Google Scholar] [CrossRef]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banat, I.M.; Banpurkar, A.G. Inhibition of pathogenic bacterial biofilms on PDMS based implants by L. acidophilus derived biosurfactant. BMC Microbiol. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Sharma, D.; Singh Saharan, B. Simultaneous production of biosurfactants and bacteriocins by probiotic lactobacillus casei MRTL3. Int. J. Microbiol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelges, H.; Tretyakova, M.; Ludwig, B. Cleansing agents containing biosurfactants and having prebiotic activity. Available online: http://www.freepatentsonline.com/y2017/0071842.html (accessed on 4 July 2020).
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Publ. Gr. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L.; Richard Gallo, C.L. Skin microbiota: A source of disease or defence? NIH Public Access. Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.P. Normal Flora. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996; Available online: https://www.ncbi.nlm.nih.gov/books/NBK7627/?report=classic (accessed on 14 November 2020).
- Tarale, P.; Gawande, S.; Jambhulkar, V. Antibiotic susceptibility profile of bacilli isolated from the skin of healthy humans. Braz. J. Microbiol. 2015, 46, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Stipcevic, T.; Piljac, T.; Isseroff, R.R. Di-rhamnolipid from Pseudomonas aeruginosa displays differential effects on human keratinocyte and fibroblast cultures. J. Dermatol. Sci. 2005, 40, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; Kitagawa, M.; Suzuki, M.; Yamamoto, S.; Sogabe, A.; Yanagidani, S.; Imura, T.; Fukuoka, T.; Kitamoto, D. A yeast glycolipid biosurfactant, mannosylerythritol lipid, shows potential moisturizing activity toward cultured human skin cells: The recovery effect of MEL-a on the SDS-damaged human skin cells. J. Oleo Sci. 2009, 58, 639–642. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.J.; Maibach, H.I. Role of ceramides in barrier function of healthy and diseased skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The slippery road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef]
- Kitagawa, M.; Suzuki, M.; Yamamoto, S.; Sogabe, A.; Kitamoto, D.; Imura, T.; Fukuoka, T.; Morita, T. Biosurfactant-containing skin care cosmetic and skin roughness-improving agent. Available online: http://www.freepatentsonline.com/y2010/0004472.html (accessed on 4 July 2020).
- Yamamoto, S.; Morita, T.; Fukuoka, T.; Imura, T.; Yanagidani, S.; Sogabe, A.; Kitamoto, D.; Kitagawa, M. The moisturizing effects of glycolipid biosurfactants, mannosylerythritol lipids, on human skin. J. Oleo Sci. 2012, 61, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.-C.; Chen, C.-Y.; Wang, T.-C.; Chen, Y.-S. Characterization of Biosurfactant from a Diesel-oil Degradation Bacterium and Application Potential in Beauty Care Products. ICBEE 2011, 20, 114–118. [Google Scholar]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Fr. 2011, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kevin Heard, L.; Chen, X.; Bollag, W.B. Aquaporins in the skin. In Advances in Experimental Medicine and Biology; Springer New York LLC.: New York, NY, USA, 2017; Volume 969, pp. 173–191. [Google Scholar]
- Ikarashi, N.; Kon, R.; Kaneko, M.; Mizukami, N.; Kusunoki, Y.; Sugiyama, K. Relationship between aging-related skin dryness and aquaporins. Int. J. Mol. Sci. 2017, 18, 1559. [Google Scholar] [CrossRef]
- Bae, I.H.; Lee, S.H.; Oh, S.; Choi, H.; Marinho, P.A.; Yoo, J.W.; Ko, J.Y.; Lee, E.S.; Lee, T.R.; Lee, C.S.; et al. Mannosylerythritol lipids ameliorate ultraviolet A-induced aquaporin-3 downregulation by suppressing c-Jun N-terminal kinase phosphorylation in cultured human keratinocytes. Korean J. Physiol. Pharmacol. 2019, 23, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Glycolipid biosurfactants, mannosylerythritol lipids, show antioxidant and protective effects against H 2O 2-induced oxidative stress in cultured human skin fibroblasts. J. Oleo Sci. 2012, 61, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Yoo, J.W.; Hwang, Y.K.; Bin, S.; Kim, Y.J.; Lee, J.H. Skin whitening composition containing mannosylerythritol lipid 2019. Available online: http://www.freepatentsonline.com/y2019/0231668.html (accessed on 4 July 2020).
- Irfan-Maqsood, M.; Seddiq-Shams, M. Rhamnolipids: Well-Characterized Glycolipids with Potential Broad Applicability as Biosurfactants. Ind. Biotechnol. 2014, 10, 285–291. [Google Scholar] [CrossRef]
- Murphrey, M.B.; Miao, J.H.; Zito, P.M. Histology, Stratum Corneum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Orton, D.I.; Wilkinson, J.D. Cosmetic allergy: Incidence, diagnosis, and management. Am. J. Clin. Dermatol. 2004, 5, 327–337. [Google Scholar] [CrossRef]
- Zirwas, M.J.; Moennich, J. Antiperspirant and deodorant allergy: Diagnosis and management. J. Clin. Aesthet. Dermatol. 2008, 1, 38–43. [Google Scholar] [PubMed]
- Yegambaram, M.; Manivannan, B.; Beach, T.; Halden, R. Role of Environmental Contaminants in the Etiology of Alzheimer’s Disease: A Review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German Federal Institute for Risk Assessment (Bfr). New studies on antiperspirants containing aluminium: Impairments to health unlikely as a result of aluminium uptake via the skin; Berlin, Germany. 2020. Available online: https//www.bfr.bund.de (accessed on 6 November 2020).
- Hwang, Y.-H.; Park, B.-K.; Lim, J.-H.; Kim, M.-S.; Song, I.-B.; Park, S.-C.; Yun, H.-I. Evaluation of Genetic and Developmental Toxicity of Surfactin C from Bacillus subtilis BC1212. J. Heal. Sci. 2008, 54, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Maeng, Y.; Kim, K.T.; Zhou, X.; Jin, L.; Kim, K.S.; Kim, Y.H.; Lee, S.; Park, J.H.; Chen, X.; Kong, M.; et al. A novel microbial technique for producing high-quality sophorolipids from horse oil suitable for cosmetic applications. Microb. Biotechnol. 2018, 11, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Bojar, R.A. Studying the Human Skin Microbiome Using 3D In Vitro Skin Models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar] [CrossRef]
- Henkel AG & CO Press release Another milestone reached in skin research. Available online: www.henkel.com/press (accessed on 18 June 2020).
- Wang, Y.; Tan, X.; Xi, C.; Phillips, K.S. Removal of Staphylococcus aureus from skin using a combination antibiofilm approach. NPJ Biofilms Microbiomes 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Duffy, E.; De Guzman, K.; Wallace, R.; Murphy, R.; Morrin, A. cosmetics Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics 2017, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- De Wever, B.; Petersohn, D.; Mewes, K.R. Overview of human three-dimensional (3D) skin models used for dermal toxicity assessment. Househ. Pers. Care Today 2013, 8, 18–23. [Google Scholar]
- MatTek Life Sciences EpiDermFT in vitro 3D Tissue | MatTek Life Sciences. Available online: https://www.mattek.com/products/epidermft/ (accessed on 1 June 2020).
- Abtin, A.; Eckhart, L.; Mildner, M.; Gruber, F.; Schröder, J.-M.; Tschachler, E. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 2008, 22, 2168–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Hoffert, U.; Zimmermann, A.; Czapp, M.; Bartels, J.; Koblyakova, Y.; Gläser, R.; Schröder, J.M.; Gerstel, U. Flagellin delivery by Pseudomonas aeruginosa rhamnolipids induces the antimicrobial protein psoriasin in human skin. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthil Balan, S.; Ganesh Kumar, C.; Jayalakshmi, S. Physicochemical, structural and biological evaluation of Cybersan (trigalactomargarate), a new glycolipid biosurfactant produced by a marine yeast, Cyberlindnera saturnus strain SBPN-27. Process Biochem. 2019, 80, 171–180. [Google Scholar] [CrossRef]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. Adv. Exp. Med. Biol. 2010, 672, 135–145. [Google Scholar] [CrossRef]
- Ahmadi-Ashtiani, H.R.; Baldisserotto, A.; Cesa, E.; Manfredini, S.; Zadeh, H.S.; Gorab, M.G.; Khanahmadi, M.; Zakizadeh, S.; Buso, P.; Vertuani, S. Microbial biosurfactants as key multifunctional ingredients for sustainable cosmetics. Cosmetics 2020, 7, 46. [Google Scholar] [CrossRef]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Mohammad Ganji, S. Cytotoxic effects of mono- and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef]
- Farris, P. Are Skincare Products with Probiotics Worth the Hype? Available online: https://www.dermatologytimes.com/view/are-skincare-products-probiotics-worth-hype (accessed on 22 June 2020).
- Mantzourani, I.; Nouska, C.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Panayiotidis, M.I.; Galanis, A.; Plessas, S. Production of a novel functional fruit beverage consisting of cornelian cherry juice and probiotic bacteria. Antioxidants 2018, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Matejuk, A. Skin Immunity. Arch. Immunol. Ther. Exp. (Warsz) 2018, 66, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]



| Skin Model | Features | Functions (Tests) | Longevity (Days) | Supplier | References |
|---|---|---|---|---|---|
| Labskin | Polymerized fibrin dermal equivalent, air exposed and well- differentiated epidermis | Studies interaction between skin and its microbiome, antimicrobial testing, etc. | 10–14 | Innoven Ltd.,York, England UK | [141,145] |
| Episkin™ | Composed of collagen lattice with human fibroblast, surface overlay of human differential epidermis (type IV collagen) | Toxicological assessment of chemicals and products, skin corrosion, etc. | 14 | SkinEthic Labs., Lyon, France | [146] |
| EpiDermFT™ | Full-thickness model with keratin 5, keratin 10 and involucrin, mitotically- and metabolically-active layers, dry surface | Wound healing, skin hydration, anti-aging | 14 | MatTek Life Sciences, Ashland, MA, USA | [147] |
| MelanoDerm™ | Human-derived epidermal keratinocyte and melanocyte, highly differentiated epidermis | Skin pigmentation, lighting efficacy of cosmetic formulations | 14 | MatTek Life Sciences, Ashland, MA, USA | [147] |
| Phenion™ FT LONG-LIFE skin model | Human skin fibroblast and keratinocyte from single donor, full-thickness skin model (3 mm) | Toxicological assessment of chemicals and products, wound healing, skin permeability, drug delivery, etc. | 50 | Henkel AG CO., Dusseldorf, Germany | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. https://doi.org/10.3390/pharmaceutics12111099
Adu SA, Naughton PJ, Marchant R, Banat IM. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics. 2020; 12(11):1099. https://doi.org/10.3390/pharmaceutics12111099
Chicago/Turabian StyleAdu, Simms A., Patrick J. Naughton, Roger Marchant, and Ibrahim M. Banat. 2020. "Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations" Pharmaceutics 12, no. 11: 1099. https://doi.org/10.3390/pharmaceutics12111099
APA StyleAdu, S. A., Naughton, P. J., Marchant, R., & Banat, I. M. (2020). Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics, 12(11), 1099. https://doi.org/10.3390/pharmaceutics12111099