A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Data Collection
2.2. Qualitative Analysis
2.3. Ethics and Informed Consent
3. Results
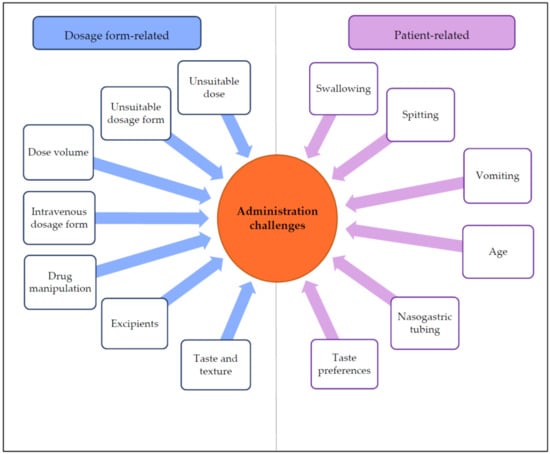
3.1. Drug Administration Challenges
3.1.1. Dosage Form-Related Administration Challenges
3.1.2. Patient-Related Administration Challenges
3.2. Suitable Dosage Forms for Pediatrics
3.3. Factors Promoting Successful Drug Administration at Hospital Wards
3.4. Factors Promoting Successful Drug Administration after Patient Discharge
3.5. Roles and Cooperation of Healthcare Professionals
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimland, E.; Nydert, P.; Odlind, V.; Böttiger, Y.; Lindemalm, S. Paediatric drug use with focus on off-label prescriptions at Swedish hospitals—A nationwide study. Acta Paediatr. 2012, 101, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Sivén, M.; Kovanen, S.; Siirola, O.; Hepojoki, T.; Isokirmo, S.; Laihanen, N.; Eränen, T.; Pellinen, J.; Juppo, A.M. Challenge of paediatric compounding to solid dosage forms sachets and hard capsules—Finnish perspective. J. Pharm. Pharm. 2017, 69, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards printed pediatric medicines in hospital pharmacies: Comparison of 2d and 3d-printed orodispersiblewarfarin films with conventional oral powders in unit dose sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef] [Green Version]
- Ivanovska, V.; Rademaker, C.M.A.; Van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Sam, T.; Ernest, T.B.; Walsh, J.; Williams, J.L. A benefit/risk approach towards selecting appropriate pharmaceutical dosage forms—An application for paediatric dosage form selection. Int. J. Pharm. 2012, 435, 115–123. [Google Scholar] [CrossRef]
- Whittaker, A.; Currie, A.E.; Turner, M.A.; Field, D.J.; Mulla, H.; Pandya, H.C. Toxic additives in medication for preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F236–F240. [Google Scholar] [CrossRef] [Green Version]
- Ernest, T.B.; Elder, D.P.; Martini, L.G.; Roberts, M.; Ford, J.L. Developing paediatric medicines: Identifying the needs and recognizing the challenges. J. Pharm. Pharm. 2007, 59, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Valeur, K.S.; Holst, H.; Allegaert, K. Excipients in Neonatal Medicinal Products: Never Prescribed, Commonly Administered. Pharm. Med. 2018, 32, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Palop, B.; Movilla Polanco, E.; Cañete Ramirez, C.; Cabañas Poy, M.J. Harmful excipients in medicines for neonates in Spain. Int. J. Clin. Pharm. 2016, 38, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Valeur, K.S.; Hertel, S.A.; Lundstrøm, K.E.; Holst, H. The Cumulative Daily Tolerance Levels of Potentially Toxic Excipients Ethanol and Propylene Glycol Are Commonly Exceeded in Neonates and Infants. Basic Clin. Pharm. Toxicol. 2018, 122, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyami, H.; Koner, J.; Huynh, C.; Terry, D.; Mohammed, A.R. Current opinions and recommendations of paediatric healthcare professionals—The importance of tablets: Emerging orally disintegrating versus traditional tablets. Plos One 2018, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lajoinie, A.; Henin, E.; Nguyen, K.A.; Malik, S.; Mimouni, Y.; Sapori, J.M.; Bréant, V.; Cochat, P.; Kassai, B. Oral drug dosage forms administered to hospitalized children: Analysis of 117,665 oral administrations in a French paediatric hospital over a 1-year period. Int. J. Pharm. 2016, 500, 336–344. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of uncoated mini-tablets in neonates—A randomized controlled trial. J. Pediatr. 2015, 167, 893–896. [Google Scholar] [CrossRef]
- Van Riet-Nales, D.A.; Ferreira, J.A.; Schobben, A.F.A.M.; de Neef, B.J.; Egberts, T.C.G.; Rademaker, C.M.A. Methods of administering oral formulations and child acceptability. Int. J. Pharm. 2015, 491, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Venables, R.; Stirling, H.; Batchelor, H.; Marriott, J. Problems with oral formulations prescribed to children: A focus group study of healthcare professionals. Int. J. Clin. Pharm. 2015, 37, 1057–1067. [Google Scholar] [CrossRef]
- Rabiee, F. Focus-group interview and data analysis. Proc. Nutr. Soc. 2004, 63, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Akram, G.; Mullen, A.B. Mixing medication into foodstuffs: Identifying the issues for paediatric nurses. Int. J. Nurs. Pract. 2015, 21, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.W.; Varker, A.S.; Karlage, K.; Myrdal, P.B. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J. Manag. Care Pharm. 2009, 15, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Helmy, S.A. Tablet splitting: Is it worthwhile? Analysis of drug content and weight uniformity for half tablets of 16 commonly used medications in the outpatient setting. J. Manag. Care Spec. Pharm. 2015, 21, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madathilethu, J.; Roberts, M.; Peak, M.; Blair, J.; Prescott, R.; Ford, J.L. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr. Open 2018, 2, e000198. [Google Scholar] [CrossRef]
- Richey, R.H.; Hughes, C.; Craig, J.V.; Shah, U.U.; Ford, J.L.; Barker, C.E.; Peak, M.; Nunn, A.J.; Turner, M.A. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int. J. Pharm. 2017, 518, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Verrue, C.; Mehuys, E.; Boussery, K.; Remon, J.P.; Petrovic, M. Tablet-splitting: A common yet not so innocent practice. J. Adv. Nurs. 2011, 67, 26–32. [Google Scholar] [CrossRef]
- Manrique, Y.J.; Lee, D.J.; Islam, F.; Nissen, L.M.; Cichero, J.A.Y.; Stokes, J.R.; Steadman, K.J. Crushed tablets: Does the administration of food vehicles and thickened fluids to aid medication swallowing alter drug release? J. Pharm. Pharm. Sci. 2014, 17, 207–219. [Google Scholar] [CrossRef]
- Trofimiuk, M.; Wasilewska, K.; Winnicka, K. How to modify drug release in paediatric dosage forms? Novel technologies and modern approaches with regard to children’s population. Int. J. Mol. Sci. 2019, 20, 3200. [Google Scholar] [CrossRef] [Green Version]
- Salman, S.; Tang, E.K.Y.; Cheung, L.C.; Nguyen, M.N.; Sommerfield, D.; Slevin, L.; Lim, L.Y.; von Ungern Sternberg, B.S. A novel, palatable paediatric oral formulation of midazolam: Pharmacokinetics, tolerability, efficacy and safety. Anaesthesia 2018, 73, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Thabet, Y.; Walsh, J.; Breitkreutz, J. Flexible and precise dosing of enalapril maleate for all paediatric age groups utilizing orodispersible minitablets. Int. J. Pharm. 2018, 541, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, M.; Stegemann, S.; Hsiao, W.-K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, S.A.; Hird-McCorry, L.P.; Goodman, D.M. Identification of fail points for discharging pediatric patients with new tracheostomy and ventilator. Hosp. Pediatr. 2016, 6, 552–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Themes |
|---|
Suitability of orally administered dosage forms to pediatric patients of different age
|
| Manipulation of drugs prior administration |
| Risks associated with pharmaceutical excipients |
| Variable | Physicians (n) | Nurses (n) | Pharmacists (n) |
|---|---|---|---|
| Gender | |||
| Female | 4 | 5 | 6 |
| Male | 4 | 0 | 0 |
| Total | 8 | 5 | 6 |
| Age | |||
| 20–34 | 0 | 1 | 0 |
| 35–49 | 6 | 2 | 5 |
| >50 | 2 | 2 | 1 |
| Dosage Form | Citations |
|---|---|
| Orodispersible | Some children take an orodispersible tablet directly into the mouth, afterwards rinsing with water or milk |
| You can give orodispersible tablets to babies who eat purees | |
| Orodispersible tablets are of course probably relatively pleasant to take | |
| Liquid or suspension | You can administer different doses of oral liquids or suspensions |
| It is easy to adjust the dose | |
| A liquid is the best alternative for the smallest children |
| Professional skills of the nurse | Skillful administration techniques |
| Knowledge about useful manipulation methods | |
| Knowledge about the preferences of each individual child | |
| Firm guidance | |
| Manipulation of drug products | Cutting a tablet into smaller pieces |
| Crushing of tablets | |
| Covering a bad taste of drug with juice or juice concentrate, glucose solution, water with added lemon concentrate, milk, fruit purees or jam (especially raspberry jam is good if the drug is in small pieces) | |
| Ex tempore manufacturing of dose powders from commercial drug products | |
| Dispersing of (crushed) tablets and dose powders before administration | |
| Use of administration aids | Coating (Medcoat®) with good taste to cover the drug and facilitate swallowing of tablets, as a whole or in halves or pieces, or capsules. |
| Consulting different sources of information | Transferring information amongst nurses |
| Consulting a clinical pharmacist | |
| Consulting the hospital pharmacy | |
| Reading the package information leaflet or summary of product characteristics | |
| Selecting the most appropriate drug product for the child | Ex tempore manufacturing of oral liquids or suspensions |
| Choosing the more viscous alternative of two liquid formulations | |
| Considering the total volume of liquid formulations to be administered | |
| Using orodispersible tablets and dispersing them with water in a small spoon | |
| Taking into account possible risks with respect to excipients |
| Profession | Role |
|---|---|
| Physician | Prescribes the active pharmaceutical ingredient, dose and route of administration. Does not intervene in the choice of dosage form. |
| Physician and nurse decide the route of administration together | |
| Nurse | Informs physician which oral dosage form an individual child can take |
| Decides which oral dosage form to administer to the child | |
| Consults physician if problems occur | |
| Knows what gimmicks to use in drug administration | |
| Gives feedback to physician about medication administration | |
| Clinical pharmacist | Discusses choice of dosage form with nurse |
| Gives advice in drug manipulation and administration matters |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children. Pharmaceutics 2020, 12, 109. https://doi.org/10.3390/pharmaceutics12020109
Rautamo M, Kvarnström K, Sivén M, Airaksinen M, Lahdenne P, Sandler N. A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children. Pharmaceutics. 2020; 12(2):109. https://doi.org/10.3390/pharmaceutics12020109
Chicago/Turabian StyleRautamo, Maria, Kirsi Kvarnström, Mia Sivén, Marja Airaksinen, Pekka Lahdenne, and Niklas Sandler. 2020. "A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children" Pharmaceutics 12, no. 2: 109. https://doi.org/10.3390/pharmaceutics12020109
APA StyleRautamo, M., Kvarnström, K., Sivén, M., Airaksinen, M., Lahdenne, P., & Sandler, N. (2020). A Focus Group Study about Oral Drug Administration Practices at Hospital Wards—Aspects to Consider in Drug Development of Age-Appropriate Formulations for Children. Pharmaceutics, 12(2), 109. https://doi.org/10.3390/pharmaceutics12020109