Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing
Abstract
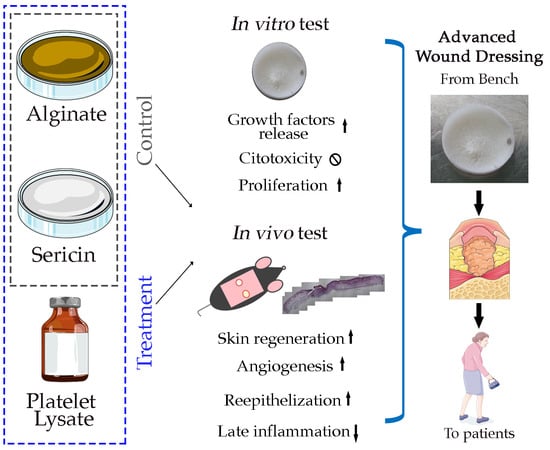
:1. Introduction
2. Materials and Methods
2.1. Biomembranes Preparation
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. In Vitro Analysis
2.3.1. Growth Factors Release
2.3.2. Cell Cultures
2.3.3. Cell Viability Assay
2.3.4. SS and PL Protective Effect against Oxidative Stress
2.3.5. BrdU Assay
2.3.6. Western Blotting
2.4. In Vivo Mouse Wound Healing Model
2.5. Histology
2.6. Statistical Analysis
3. Results
3.1. FTIR Analyses of Single Components and Biomembranes
3.2. Proteins and Growth Factors Released from the Biomembranes
3.3. Biomembrane Biocompatibility
3.4. Protection against Oxidative Stress due to the Biomembrane Components
3.5. Biomembrane Effect on the Healing of a Mouse Excisional Wound Model
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef] [PubMed]
- Report Global Wound Dressings Market 2018–2022; TechNavio (Infiniti Research Ltd.): London, UK, 2018.
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraglia, A.; Todeschi, M.R.; Papait, A.; Poggi, A.; Spanò, R.; Strada, P.; Cancedda, R.; Mastrogiacomo, M. Combined platelet and plasma derivatives enhance proliferation of stem/progenitor cells maintaining their differentiation potential. Cytotherapy 2015, 17, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Spanò, R.; Muraglia, A.; Todeschi, M.R.; Nardini, M.; Strada, P.; Cancedda, R.; Mastrogiacomo, M. Platelet-rich plasma-based bioactive membrane as a new advanced wound care tool. J. Tissue Eng. Regen. Med. 2018, 12, e82–e96. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef]
- Morimoto, N.; Yoshimura, K.; Niimi, M.; Ito, T.; Aya, R.; Fujitaka, J.; Tada, H.; Teramukai, S.; Murayama, T.; Toyooka, C.; et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: Clinical trial for chronic skin ulcers. Tissue Eng. Part A 2013, 19, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, N.; Anniboletti, T.; Carlesimo, B.; Onesti, M.G. Clinical application of autologous three-cellular cultured skin substitutes based on esterified hyaluronic acid scaffold: Our experience. Vivo Athens Greece 2009, 23, 991–1003. [Google Scholar]
- Uccioli, L.; Giurato, L.; Ruotolo, V.; Ciavarella, A.; Grimaldi, M.S.; Piaggesi, A.; Teobaldi, I.; Ricci, L.; Scionti, L.; Vermigli, C.; et al. Two-step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: Results of a multicenter, randomized controlled clinical trial with long-term follow-up. Int. J. Low. Extrem. Wounds 2011, 10, 80–85. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Faragò, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Faragò, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-α blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalathevan, P.; Ooi, P.S.; Loo, Y.L. Silk-Based Biomaterials in Cutaneous Wound Healing: A Systematic Review. Adv. Skin Wound Care 2018, 31, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Luplertlop, N.; Kanjanapruthipong, T.; Ampawong, S. Effect of urea-extracted sericin on melanogenesis: Potential applications in post-inflammatory hyperpigmentation. Biol. Res. 2018, 51, 54. [Google Scholar] [CrossRef] [PubMed]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Faragò, S.; Lucconi, G.; Perteghella, S.; Vigani, B.; Tripodo, G.; Sorrenti, M.; Catenacci, L.; Boschi, A.; Faustini, M.; Vigo, D.; et al. A dry powder formulation from silk fibroin microspheres as a topical auto-gelling device. Pharm. Dev. Technol. 2016, 21, 453–462. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [Green Version]
- Langan, T.J.; Rodgers, K.R.; Chou, R.C. Synchronization of Mammalian Cell Cultures by Serum Deprivation. Methods Mol. Biol. 2017, 1524, 97–105. [Google Scholar] [PubMed]
- Galiano, R.D.; Michaels, J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.; Golden, B.; Sullivan, M.; Botros, S.M.; Miller, J.-J.R.; Alshahrour, A.; Goldberg, R.P.; Sand, P.K. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007, 15, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [Green Version]
- Zhaorigetu, S.; Sasaki, M.; Kato, N. Consumption of sericin suppresses colon oxidative stress and aberrant crypt foci in 1,2-dimethylhydrazine-treated rats by colon undigested sericin. J. Nutr. Sci. Vitaminol. 2007, 53, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Zhaorigetu, S.; Sasaki, M.; Watanabe, H.; Kato, N. Supplemental silk protein, sericin, suppresses colon tumorigenesis in 1,2-dimethylhydrazine-treated mice by reducing oxidative stress and cell proliferation. Biosci. Biotechnol. Biochem. 2001, 65, 2181–2186. [Google Scholar] [CrossRef] [Green Version]
- Sano, M.; Tamada, Y.; Niwa, K.; Morita, T.; Yoshino, G. Sulfated sericin is a novel anticoagulant influencing the blood coagulation cascade. J. Biomater. Sci. Polym. Ed. 2009, 20, 773–783. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Wu, A.; Wang, J.; Ge, C.; Sun, Z. Self-assembled silk fibroin nanoparticles loaded with binary drugs in the treatment of breast carcinoma. Int. J. Nanomed. 2016, 11, 4373–4380. [Google Scholar]
- Padamwar, M.N.; Pawar, A.P. Silk Sericin and its Applications: A Review. J. Sci. Ind. Res. 2004, 20, 323–329. [Google Scholar]
- Takechi, T.; Takamura, H. Development of Bread Supplemented with the Silk Protein Sericin. Food Sci. Technol. Res. 2014, 20, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Tao, G.; Wang, Y.; Cai, R.; Guo, P.; Chen, L.; Zuo, H.; Zhao, P.; Xia, Q. In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 509–516. [Google Scholar] [CrossRef]
- Akeda, K.; Yamada, J.; Linn, E.T.; Sudo, A.; Masuda, K. Platelet-rich plasma in the management of chronic low back pain: A critical review. J. Pain Res. 2019, 12, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Andia, I.; Abate, M. Platelet-rich plasma: Combinational treatment modalities for musculoskeletal conditions. Front. Med. 2018, 12, 139–152. [Google Scholar] [CrossRef]
- Li, T.; Ma, Y.; Wang, M.; Wang, T.; Wei, J.; Ren, R.; He, M.; Wang, G.; Boey, J.; Armstrong, D.G.; et al. Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect. Drug Resist. 2019, 12, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Merchán, W.H.; Gómez, L.A.; Chasoy, M.E.; Alfonso-Rodríguez, C.A.; Muñoz, A.L. Platelet-rich plasma, a powerful tool in dermatology. J. Tissue Eng. Regen. Med. 2019, 13, 892–901. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Stute, N.; Holtz, K.; Bubenheim, M.; Lange, C.; Blake, F.; Zander, A.R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 2004, 32, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hirayama, F.; Wakamoto, S.; Fujihara, M.; Murahashi, H.; Sato, N.; Ikebuchi, K.; Sawada, K.; Koike, T.; Kuwabara, M.; et al. Bone marrow stromal cells prepared using AB serum and bFGF for hematopoietic stem cells expansion. Transfusion 2002, 42, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, A.; Ottonello, C.; Spanò, R.; Dozin, B.; Strada, P.; Grandizio, M.; Cancedda, R.; Mastrogiacomo, M. Biological activity of a standardized freeze-dried platelet derivative to be used as cell culture medium supplement. Platelets 2014, 25, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Backly, R.E.; Ulivi, V.; Tonachini, L.; Cancedda, R.; Descalzi, F.; Mastrogiacomo, M. Wound Healing of Human Keratinocytes Associated with a Strong Proinflammatory Response. Tissue Eng. Part A 2011, 17, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Romaldini, A.; Ulivi, V.; Nardini, M.; Mastrogiacomo, M.; Cancedda, R.; Descalzi, F. Platelet Lysate Inhibits NF-κB Activation and Induces Proliferation and an Alert State in Quiescent Human Umbilical Vein Endothelial Cells Retaining Their Differentiation Capability. Cells 2019, 8, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Tenci, M.; Del Fante, C.; Nicoletti, G.; Caramella, C. Sponge-Like Dressings Based on the Association of Chitosan and Sericin for the Treatment of Chronic Skin Ulcers. II. Loading of the Hemoderivative Platelet Lysate. J. Pharm. Sci. 2016, 105, 1188–1195. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Tripodo, G.; Mastrogiacomo, M.; Mandracchia, D.; Trapani, A.; Faragò, S.; et al. In vitro efficacy of silk sericin microparticles and platelet lysate for intervertebral disk regeneration. Int. J. Biol. Macromol. 2018, 118, 792–799. [Google Scholar] [CrossRef]
- Dash, R.; Acharya, C.; Bindu, P.C.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Papait, A.; Cancedda, R.; Mastrogiacomo, M.; Poggi, A. Allogeneic platelet-rich plasma affects monocyte differentiation to dendritic cells causing an anti-inflammatory microenvironment, putatively fostering wound healing. J. Tissue Eng. Regen. Med. 2018, 12, 30–43. [Google Scholar] [CrossRef]









| Type of Biomembrane | Name | Solution Percentage Composition (w/v) | Membrane Percentage Composition (w/w %) | ||||
|---|---|---|---|---|---|---|---|
| Alg | SS | PL | Alg | SS | PL | ||
| Control | Alg/SS | 1 | 1 | 0 | 50 | 50 | 0 |
| Treated | Alg/SS/PL | 1 | 1 | 2 | 25 | 25 | 50 |
| Treated | Alg/PL | 1 | 0 | 1 | 50 | 0 | 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. https://doi.org/10.3390/pharmaceutics12020120
Nardini M, Perteghella S, Mastracci L, Grillo F, Marrubini G, Bari E, Formica M, Gentili C, Cancedda R, Torre ML, et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics. 2020; 12(2):120. https://doi.org/10.3390/pharmaceutics12020120
Chicago/Turabian StyleNardini, Marta, Sara Perteghella, Luca Mastracci, Federica Grillo, Giorgio Marrubini, Elia Bari, Matteo Formica, Chiara Gentili, Ranieri Cancedda, Maria Luisa Torre, and et al. 2020. "Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing" Pharmaceutics 12, no. 2: 120. https://doi.org/10.3390/pharmaceutics12020120
APA StyleNardini, M., Perteghella, S., Mastracci, L., Grillo, F., Marrubini, G., Bari, E., Formica, M., Gentili, C., Cancedda, R., Torre, M. L., & Mastrogiacomo, M. (2020). Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics, 12(2), 120. https://doi.org/10.3390/pharmaceutics12020120