A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of a Decellularization Method for Porcine Skin
2.2. Evaluation of the Decellularization Efficiency
2.2.1. Histological Analysis
2.2.2. Biochemical Analysis
2.3. Preparation and Process Optimization of aECM Hydrogels
2.4. Characterization of aECM Hydrogels
2.4.1. Sol-Gel Phase Transition
2.4.2. Rheological Studies of aECM Hydrogels
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Qualitative Analysis of Collagen in aECM Hydrogels
2.5. Fabrication and In Vitro Cell Viability Test of Hydrogel Dressings
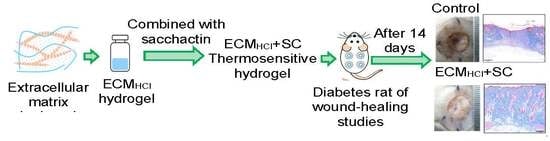
2.6. Wound-Healing Studies
2.6.1. Diabetic Rats in Wound-Healing Studies
2.6.2. Histological Analysis
3. Results and Discussion
3.1. Evaluation of the Decellularization Efficiency
3.2. Preparation and Characterization of aECM Hydrogels
3.3. In Vitro Cell Viability Test
3.4. In Vivo Wound-Healing Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schultz, G.S.; Ladwig, G.; Wysocki, A. Extracellular matrix: Review of its roles in acute and chronic wounds. World Wide Wounds 2005, 2005, 1–18. [Google Scholar]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissue and organs. Biomaterials 2006, 27, 3657–3683. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization process. Biomaterials 2011, 32, 3233–3243. [Google Scholar]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.C.; Mirmalek, S.H.; Deegan, D.B.; Baptista, P.M.; Aboushwareb, T.; Atala, A.; Yoo, J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012, 33, 7756–7764. [Google Scholar] [CrossRef]
- Oliver, R.F.; Grant, R.A.; Kent, C.M. The fate of cutaneously and subcutaneously implanted trypsin purified dermal collagen in the pig. Br. J. Exp. Pathol. 1972, 53, 540–549. [Google Scholar]
- Prasertsung, I.; Kanokpanont, S.; Bunaprasert, T.; Thanakit, V.; Darmrongsakkul, S. Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Mat. Res. Part B Appl. Biomater. 2008, 85, 210–219. [Google Scholar] [CrossRef]
- Arenas-Herrera, J.; Ko, I.; Atala, A.; Yoo, J. Decellularization for whole organ bioengineering. Biomed. Mater. 2013, 8, 014016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, R.N.; Jhan, H.J.; Liu, D.Z.; Ho, H.O.; Mao, Y.; Kohn, J.; Sheu, M.T. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng. Part C Methods 2015, 21, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Negishi, J.; Funamoto, S.; Kimura, T.; Nam, K.; Higami, T.; Kishida, A. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J. Artif. Organs 2011, 14, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Kim, S.W.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue and analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; Román, S.J. Bioadhesive functional hydrogels: Controlled release of catechol species T with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J.; Pérez-Caballer, A.; Olmeda-Lozano, M. Contribution of Bioactive Hyaluronic Acid and Gelatin to Regenerative Medicine, Methodologies of Gels Preparation and Advanced Applications. Eur. Polym. J. 2017, 95, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Kim, S.Y. Application of a paste-type acellular dermal matrix for coverage of chronic ulcerative wounds. Arch. Plast. Surg. 2018, 45, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spang, M.T.; Christman, K.L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Voytik-Harbin, S.L.; Brightman, A.O. Small intestinal submucosa: A tissue derived extracellular matrix that promotes tissue-specific growth and differentiation of cells in vitro. Tissue Eng. 1998, 4, 157–174. [Google Scholar] [CrossRef]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Kelly, J.; Tottey, S.; Daly, K.A.; Johnson, S.A.; Siu, B.F.; Reing, J.; Badylak, S.F. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng. Part A 2011, 17, 3033–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkinson, J.; Kadler, K.E.; Brass, A. Simple physical model of collagen fibrillogenesis based on diffusion limited aggregation. J. Mol. Biol. 1995, 247, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Mu, D.; Gao, F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: A systematic review and meta-analysis. Int. J. Surg. 2017, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.C.; Wu, M.H.; Chen, L.C.; Lin, H.L.; Liu, D.Z.; Ho, H.O.; Sheu, M.T. Preparation and characterization of chemically TEMPO-oxidized and mechanically disintegrated sacchachitin nanofibers (SCNF) for enhanced diabetic wound healing. Carbohydr. Polym. 2020, 229, 115507. [Google Scholar] [CrossRef]
- Chen, R.N.; Lee, L.W.; Chen, L.C.; Ho, H.O.; Lui, S.C.; Sheu, M.T.; Su, C.H. Wound-healing effect of micronized sacchachitin (mSC) nanogel on corneal epithelium. Int. J. Nanomed. 2012, 7, 4697–4706. [Google Scholar]
- Ho, H.O.; Lin, L.H.; Sheu, M.T. Characterization of collagen isolation and application of collagen gel as a drug carrier. J. Control. Release 1997, 44, 103–112. [Google Scholar] [CrossRef]
- Jhan, H.J.; Liu, J.J.; Chen, Y.C.; Liu, D.Z.; Sheu, M.T.; Ho, H.O. Novel injectable thermosensitive hydrogels for delivering hyaluronic acid-doxorubicin nanocomplexes to locally treat tumors. Nanomed. UK 2014, 10, 1263–1274. [Google Scholar] [CrossRef]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tan, F.J.; Marra, K.G.; Jan, S.S.; Liu, D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Khosravimelal, S.; Momeni, M.; Gholipur, M.; Kundu, S.C.; Gholipourmalekabadi, M. Protocols for decellularization of human amniotic membrane. Methods Cell Biol. 2020, 157, 37–47. [Google Scholar] [PubMed]
- Li, J.; Wang, M.C.; Qiao, Y.Y.; Tian, Y.Y.; Liu, J.H.; Qin, S.; Wu, W.H. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process. Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Hung, W.S.; Fang, C.L.; Su, C.H.; Lai, W.F.T.; Chang, Y.C.; Tsai, Y.H. Cytotoxicity and immunogenicity of sacchachitin and its mechanism of action on skin wound healing. J. Biomed. Mater. Res. 2001, 56, 93–100. [Google Scholar] [CrossRef]
- Ciccone, V.; Zazzetta, M.; Morbidelli, L. Comparison of the effect of two hyaluronic acid preparations on fibroblast and endothelial cell functions related to angiogenesis. Cells 2019, 8, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, J.W.; Lee, J.H.; Chung, K.J.; Kim, T.G.; Kim, Y.H.; Kim, K.J. Wound healing effects of paste type acellular dermal matrix subcutaneous injection. Arch. Plast. Surg. 2018, 45, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhu, Z.; Wu, D.W.; Gan, W.D.; Zhu, S.S.; Li, W.Q.; Tian, J.H.; Li, L.H.; Zhou, C.R.; Lu, L. Antibacterial poly(ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Lee, M.; Jun, D.; Choi, H.; Kim, J.; Shin, D. Clinical Efficacy of Acellular Dermal Matrix Paste in Treating Diabetic Foot Ulcers. Wounds 2020, 1, 50–56. [Google Scholar]
- Griffin, L.; Carter, M.J.; D’Agostino, R., Jr.; D’Agostino McGowan, L. Comparative Effectiveness of Two Collagen-containing Dressings: Oxidized Regenerated Cellulose (ORC)/Collagen/Silver-ORC Dressing Versus Ovine Collagen Extracellular Matrix. Wounds 2019, 31, E73–E76. [Google Scholar]








| Acronym | aECM (mg/mL) | Acid | 25 °C | 37 °C |
|---|---|---|---|---|
| aECMHCl,10 | 10 | 0.1 N HCl | Solution | Solution |
| aECMGA,10 | 10 | GA | Solution | Solution |
| aECMPCA,10 | 10 | PCA | Solution | Solution |
| aECMHCl,25 | 25 | 0.1 N HCl | Solution | Gel |
| aECMGA,25 | 25 | GA | Solution | Glu |
| aECMPCA,25 | 25 | PCA | Solution | Glu |
| aECMHCl,50 | 50 | 0.1 N HCl | Gel | Gel |
| aECMGA,50 | 50 | GA | Gel | Gel |
| aECMPCA,50 | 50 | PCA | Gel | Gel |
| Hydrogel Dressings | Formulations |
|---|---|
| HEC | 300 mg HEC in 20 mL ddH2O |
| aECMHCl,25 | 500 mg aECMHCl,25 in 20 mL 0.1 N HCl |
| SC | 400 mg SC in 20 mL ddH2O |
| HA | 400 mg HA in 20 mL ddH2O |
| CS | 400 mg CS in 20 mL 0.1 N glycolic acid adjusted to pH 7.0 with 2 N NaOH |
| aECMHCl,25/SC | 10 mL aECMHCl,25 + 10 mL SC |
| HA/SC | 200 mg HA in 20 mL SC |
| CS/SC | 400 mg CS in 20 mL SC |
| Treatments | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Residual DNA (ng/mg dry weight) | ||||
| PBS | 349.77 ± 8.33 | - | - | - |
| 10% FA | - | 93.43 ± 1.14 * | 90.89 ± 2.02 * | 87.16 ± 2.86 * |
| 20% FA | - | 87.84 ± 0.85 * | 87.35 ± 3.90 * | 77.32 ± 0.82 * |
| 30% FA | - | 67.01 ± 3.75 * | 58.87 ± 0.51 * | 42.95 ± 0.73 * |
| Total collagen (µg/mg dry weight) | ||||
| PBS | 715.39 ± 7.34 | - | - | - |
| 10% FA | - | 647.15 ± 18.51 | 630.45 ± 13.43 | 625.87 ± 2.38 |
| 20% FA | - | 610.25 ± 2.13 | 583.85 ± 3.90 | 566.52 ± 7.34 |
| 30% FA | - | 574.69 ± 7.46 | 567.24 ± 7.97 | 556.01 ± 5.94 |
| Glycosaminoglycan (µg/mg dry weight) | ||||
| PBS | 14.88 ± 1.21 | - | - | - |
| 10% FA | - | 10.61 ± 0.32 * | 8.97 ± 0.40 * | 9.59 ± 0.32 * |
| 20% FA | - | 9.19 ± 0.08 * | 7.79 ± 0.08 * | 6.52 ± 0.32 * |
| 30% FA | - | 7.09 ± 0.64 * | 6.86 ± 0.80 * | 5.50 ± 0.16 * |
| Wound Dressings | Scarring | H&E | MT | CD31 | Sum of Grading Score |
|---|---|---|---|---|---|
| Normal skin | 5 | 5 | 5 | 5 | 20 |
| HEC | 1 | 1 | 1 | 3 | 6 |
| ECMHCl | 4 | 3 | 3 | 4 | 14 |
| SC | 4 | 1 | 1 | 3 | 9 |
| HA | 3 | 1 | 1 | 3 | 8 |
| CS | 1 | 1 | 1 | 3 | 6 |
| ECMHCl+SC | 4 | 4 | 5 | 4 | 17 |
| HA+SC | 4 | 2 | 3 | 3 | 12 |
| CS+SC | 2 | 1 | 1 | 3 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-M.; Wang, W.; Chen, Y.-H.; Wei, P.-S.; Liu, Y.-H.; Sheu, M.-T.; Ho, H.-O. A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing. Pharmaceutics 2020, 12, 538. https://doi.org/10.3390/pharmaceutics12060538
Hsieh C-M, Wang W, Chen Y-H, Wei P-S, Liu Y-H, Sheu M-T, Ho H-O. A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing. Pharmaceutics. 2020; 12(6):538. https://doi.org/10.3390/pharmaceutics12060538
Chicago/Turabian StyleHsieh, Chien-Ming, Weu Wang, Ying-Hsuan Chen, Pu-Sheng Wei, Yu-Hsuan Liu, Ming-Thau Sheu, and Hsiu-O Ho. 2020. "A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing" Pharmaceutics 12, no. 6: 538. https://doi.org/10.3390/pharmaceutics12060538
APA StyleHsieh, C. -M., Wang, W., Chen, Y. -H., Wei, P. -S., Liu, Y. -H., Sheu, M. -T., & Ho, H. -O. (2020). A Novel Composite Hydrogel Composed of Formic Acid-Decellularized Pepsin-Soluble Extracellular Matrix Hydrogel and Sacchachitin Hydrogel as Wound Dressing to Synergistically Accelerate Diabetic Wound Healing. Pharmaceutics, 12(6), 538. https://doi.org/10.3390/pharmaceutics12060538