A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
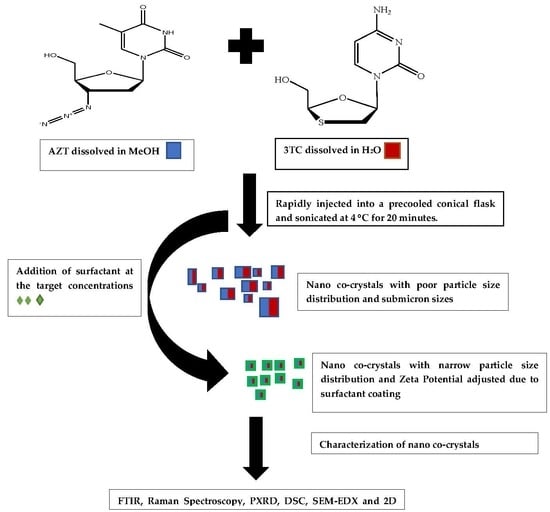
2.2.1. Preparation of Micro and Nano Co-Crystals Using a Pseudo One Solvent Bottom-Up Method
2.2.2. Particle Size Analysis
2.2.3. Zeta Potential
2.2.4. FTIR Spectroscopy
2.2.5. Raman Spectroscopy
2.2.6. Differential Scanning Calorimetry
2.2.7. Powder X-ray Diffraction (PXRD)
2.2.8. Energy Dispersive X-ray Spectroscopy Scanning Electron Microscopy
2.2.9. Preparation of Surfactant-Coated Nano Co-Crystals via a Pseudo One-Solvent Bottom-Up Method
3. Results
3.1. Co-Crystal Synthesis
3.2. Co-Crystal Characterization
3.2.1. FTIR Spectroscopy
3.2.2. Raman Spectroscopy
3.2.3. Differential Scanning Calorimetry
3.2.4. Powder X-ray Diffraction
3.2.5. Energy Dispersive X-ray Scanning Electron Microscopy (EDX-SEM)
3.3. Surfactant-Coated Co-Crystals
3.3.1. Particle Size (PS) and Polydispersity Index (PDI)
3.3.2. Zeta Potential
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV Statistics; UNAIDS: Geneva, Switzerland, 2019. [Google Scholar]
- Subramanian, S. Manifestations of noncovalent bonding in the solid state. 6.’ [~,(cyclam)]~+ (cyclam = 1,4,8,11 mtetraazacyclotetra- decane) as a template for crystal engineering of network hydrogen- bonded solids. Can. J. Chem. 1995, 73, 414–424. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Aakeroy, C.B.; Aakeroy, A.; Sinha, A.S. Co-Crystals: Introduction and Scope; Royal Society of Chemistry: London, UK, 2018; Volume 11. [Google Scholar]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. Int. Ed. 2011, 50, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G. Pharmaceutical cocrystals: The coming wave of new drug substances. J. Pharm. Sci. 2013, 102, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G. Cocrystal Systems of Pharmaceutical Interest: 2010. Cryst. Growth Des. 2011, 36, 361–381. [Google Scholar] [CrossRef]
- Sekhon, B. Pharmaceutical co-crystals—A review. ARS Pharm. 2009, 150, 99–117. [Google Scholar]
- Gao, Y.; Zu, H.; Zhang, J. Enhanced dissolution and stability of adefovir dipivoxil by cocrystal formation. J. Pharm. Pharmacol. 2011, 63, 483–490. [Google Scholar] [CrossRef]
- Yadav, A.; Shete, A.; Dabke, A.; Kulkarni, P.; Sakhare, S. Co-crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J. Pharm. Sci. 2009, 71, 359. [Google Scholar] [CrossRef] [Green Version]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. B 2018, 8, 733–755. [Google Scholar] [CrossRef]
- Trask, A.V. An overview of pharmaceutical cocrystals as intellectual property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Sinha, B.; Muller, R.H.; Moschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 8, 384–392. [Google Scholar] [CrossRef] [PubMed]
- De Waard, H.; Frijlink, H.W.; Hinrichs, W.L.J. Bottom-up preparation techniques for nanocrystals of lipophilic drugs. Pharm. Res. 2011, 28, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Quan, P.; Piao, H.; Piao, H.; Sun, S.; Yin, Y.; Cui, F. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur. J. Pharm. Sci. 2010, 40, 325–334. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, Z.H.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Sander, J.R.G.; Bučar, D.K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Pharmaceutical nano-cocrystals: Sonochemical synthesis by solvent selection and use of a surfactant. Angew. Chem. Int. Ed. 2010, 49, 7284–7288. [Google Scholar] [CrossRef]
- Macgillivray, L.R.; Sander, J.R.; Bucar, D.K.; Elacqua, E.; Zhang, G.; Henry, R. Sonochemical synthesis of nano-cocrystals Boronic ester-based adducts. Proc. Meet. Acoust. 2013, 19, 45090. [Google Scholar]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Zhang, B.; Qi, D.; Guo, P.; Li, N.; Liu, Z. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J. Pharm. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO/TS 80004-2—Nanotechnologies—Vocabulary—Part 2:Nano-objects 2015; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Morsy, S.M.I. Role of surfactants in nanotechnology and their applications. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 237–260. [Google Scholar]
- Karashima, M.; Kimoto, K.; Yamamoto, K.; Kojima, T.; Ikeda, Y. A novel solubilization technique for poorly soluble drugs through the integration of nanocrystal and cocrystal technologies. Eur. J. Pharm. Biopharm. 2016, 107, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Teeranachaideekul, V.; Junyaprasert, V.B.; Souto, E.B.; Müller, R.H. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology. Int. J. Pharm. 2008, 354, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shao, J.; Fu, Q.; Li, J.; Sun, J.; He, Z. Spray-dried nanocrystals for a highly hydrophobic drug: Increased drug loading, enhanced redispersity, and improved oral bioavailability. Int. J. Pharm. 2017, 516, 372–379. [Google Scholar] [CrossRef]
- De Smet, L.; Saerens, L.; De Beer, T.; Carleer, R.; Adriaensens, P.; Van Bocxlaer, J.; Vervaet, C.; Remon, J.P. Formulation of itraconazole nanococrystals and evaluation of their bioavailability in dogs. Eur. J. Pharm. Biopharm. 2014, 87, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhang, X.; Zhang, T.; Wang, H.; Wu, B. Design and evaluation of injectable niclosamide nanocrystals prepared by wet media milling technique. Drug Dev. Ind. Pharm. 2015, 41, 1416–1424. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Ferreira, N.R.; Fangueiro, J.F.; Santos, A.C.; Veiga, F.J.; Cabral, C.; Silva, A.M.; Souto, E.B. Ibuprofen nanocrystals developed by 22 factorial design experiment: A new approach for poorly water-soluble drugs. Saudi Pharm. J. 2017, 25, 1117–1124. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of surfactants and drug load on physico-mechanical and dissolution properties of nanocrystalline tadalafil-loaded oral films. Eur. J. Pharm. Sci. 2017, 109, 372–380. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Samanta, M.K.; Raichur, A.M. Dual-Drug Delivery System Based on In Situ Gel-Forming Nanosuspension of Forskolin to Enhance Antiglaucoma Efficacy. AAPS PharmSciTech 2010, 11, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Dash, P.K.; Gendelman, H.E.; Roy, U.; Balkundi, S.; Mosley, R.L.; Gelbard, H.A.; Mcmillan, J.; Gorantla, S.; Poluektova, L.Y. Long-acting NanoART Elicits Potent Antiretroviral and Neuroprotective Responses in HIV-1 Infected Humanized Mice. AIDS 2012, 26, 2135–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahesh, K.V.; Singh, S.K.; Gulati, M. A comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014, 256, 436–449. [Google Scholar] [CrossRef]
- Sohn, J.S.; Yoon, D.S.; Sohn, J.Y.; Park, J.S.; Choi, J.S. Development and evaluation of targeting ligands surface modified paclitaxel nanocrystals. Mater. Sci. Eng. C 2017, 72, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Kao, J.P.Y.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Liu, M.; Hong, C.; Li, G.; Ma, P.; Xie, Y. The generation of myricetin-nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology 2016, 27, 395601. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Macgillivray, L.R. Preparation and Reactivity of Nanocrystalline Cocrystals Formed via Sonocrystallization. J. Am. Chem. Soc. 2007, 129, 32. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-Crystals of the Anti-HIV Drugs Lamivudine and Zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- Lupin Limited. WO 2009/116055 Al 2009; Lupin Limited: Mumbaid, India, 2009. [Google Scholar]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Müllertz, A.; Perrie, Y.; Rades, T. Advances in Delivery Science and Technology: Analytical Techniques in the Pharmaceutical Sciences; Rathbone, M.J., Ed.; Springer Science and Business Media LLC: New York, NY, USA, 2016; ISBN 9781493940271. [Google Scholar]
- Ebnesajjad, S. Surface and Material Characterization Techniques; Ebnesajjad, S., Ed.; Andrew William Applied Science Publishers: Oxford, UK, 2011; ISBN 978-1-4377-4461-3. [Google Scholar]
- Brink, G. Infrared Studies of Water in Crystalline Hydrates: Ba(ClO3)2·H2O. Appl. Spectrosc. 1976, 30, 630–631. [Google Scholar] [CrossRef]
- Falk, M.; Huang, C.-H.; Knop, O. Infrared Spectra of Water in Crystalline Hydrates: KSnCl 3H2O, an Untypical Monohydrate. Can. J. Chem. 2006, 52, 2928–2931. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.G.; Vianna-Soares, C.D.; Righi, A.; Pinheiro, M.V.B.; Flores, M.Z.S.; Bezerra, E.M.; Freire, V.N.; Lemos, V.; Caetano, E.W.S.; Cavada, B.S. Identification of lamivudine conformers by Raman scattering measurements and quantum chemical calculations. J. Pharm. Biomed. Anal. 2007, 43, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, H.; Xue, J.; Tang, W.; Fang, H.; Zhang, Q.; Li, Y.; Hong, Z. Vibrational spectroscopic study of polymorphism and polymorphic transformation of the anti-viral drug lamivudine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Mircescu, N.E.; Varvescu, A.; Herman, K.; Chiş, V.; Leopold, N. Surface-enhanced Raman and DFT study on zidovudine. Spectroscopy 2011, 26, 311–315. [Google Scholar] [CrossRef]
- Palermo, E.F.; Chiu, J. Critical review of methods for the determination of purity by differential scanning calorimetry *. Thermochim. Acta 1976, 14, 1–12. [Google Scholar] [CrossRef]
- Lai, S.L.; Guo, J.Y.; Petrova, V.; Ramanath, G.; Allen, L.H. Size-Dependent Melting Properties of Small Tin Particles: Nanocalorimetric Measurements. Phys. Rev. Lett. 1996, 77, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Kusche, R.; von Issendorff, B.; Haberland, H. Irregular variations in the melting point of size-selected atomic clusters. Nature 1998, 393, 238–240. [Google Scholar] [CrossRef]
- Chogale, M.M.; Ghodake, V.N.; Patravale, V.B. Performance parameters and characterizations of nanocrystals: A brief review. Pharmaceutics 2016, 8, 26. [Google Scholar] [CrossRef]
- Kocbek, P.; Baumgartner, S.; Kristl, J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int. J. Pharm. 2006, 312, 179–186. [Google Scholar] [CrossRef]
- Kamb, W.B. Theory of Preferred Crystal Orientation Developed. J. Geol. 1958, 67, 153–170. [Google Scholar] [CrossRef]
- Lang, A.R. X-ray diffraction procedures for polycrystal-line and amorphous materials. Acta Metall. 1956, 4, 102. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Dave, R.N. Controlling Particle Size of a Poorly Water-Soluble Drug Using Ultrasound and Stabilizers in Antisolvent Precipitation. Ind. Eng. Chem. Res. 2009, 48, 7581–7593. [Google Scholar] [CrossRef]
- Rachmawati, H.; Al Shaal, L.; Muller, R.H.; Keck, C.M.; Shaal, L.A.; Müller, R.H.; Keck, C.M. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing agents for drug nanocrystals: Effect on bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-H.; Koelsch, P.; Weidner, T.; Wagner, M.S.; Castner, D.G. Sodium Dodecyl Sulfate Adsorption onto Positively Charged Surfaces: Monolayer Formation With Opposing Headgroup Orientations. Langmuir 2013, 29, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Keck, C.M. Cyclosporine Nanosuspensions: Optimised Size Characterisation and Oral Formulations; Freie Universitat Berlin: Berlin, Germany, 2006. [Google Scholar]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Qi, J.; Ding, N.; Dong, X.; Zhao, W.; Lu, Y.; Wang, C.; Wu, W. Tracking translocation of self-discriminating curcumin hybrid nanocrystals following intravenous delivery. Int. J. Pharm. 2018, 546, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, M.E.; Hotze, M.A.; Johnston, K.P.; Williams, R.O. Drug nanoparticles by antisolvent precipitation: Mixing energy versus surfactant stabilization. Langmuir 2006, 22, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ng, W.K.; Shen, S.; Kim, S.; Tan, R.B.H. Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation. Int. J. Pharm. 2009, 375, 84–88. [Google Scholar] [CrossRef] [PubMed]







| Std. Run | Run No. | Surfactant | Concentration % w/v |
|---|---|---|---|
| 8 | 1 | TPGS 1000 | 1 |
| 2 | 2 | SDS | 0.5 |
| 11 | 3 | Span 80 | 2 |
| 10 | 4 | SDS | 2 |
| 7 | 5 | Span 80 | 1 |
| 5 | 6 | Tween 80 | 1 |
| 9 | 7 | Tween 80 | 2 |
| 4 | 8 | TPGS 1000 | 0.5 |
| 12 | 9 | TPGS 1000 | 2 |
| 3 | 10 | Span 80 | 0.5 |
| 1 | 11 | Tween 80 | 0.5 |
| 6 | 12 | SDS | 1 |
| Element | Micro Co-Crystal | Nano Co-Crystal |
|---|---|---|
| Atomic % | Atomic % | |
| CK | 48.26 ± 0.52 | 49.67 ± 1.21 |
| NK | 20.75 ± 0.87 | 20.81 ± 0.94 |
| OK | 29.96 ± 0.73 | 28.53 ± 1.02 |
| SK | 1.03 ± 0.09 | 1.00 ± 0.03 |
| Std. Run | Run No. | Surfactant | Concentration % w/v | PS nm | PDI | ZP mV |
|---|---|---|---|---|---|---|
| 8 | 1 | TPGS 1000 | 1 | 200.6 ± 28.91 | 0.467 ± 0.077 | −2.57 ± 0.63 |
| 2 | 2 | SDS | 0.5 | 1099 ± 166.10 | 0.811 ± 0.051 | −18.2 ± 2.35 |
| 11 | 3 | Span 80 | 2 | 351.5 ± 21.19 | 0.288 ± 0.078 | −4.2 ± 1.22 |
| 10 | 4 | SDS | 2 | 182.1 ± 11.60 | 0.331 ± 0.086 | −42.5 ± 3.41 |
| 7 | 5 | Span 80 | 1 | 736.7 ± 77.15 | 0.663 ± 0.022 | −7.1 ± 1.13 |
| 5 | 6 | Tween 80 | 1 | 356 ± 42.09 | 0.357 ± 0.008 | −1.04 ± 0.35 |
| 9 | 7 | Tween 80 | 2 | 360 ± 88.67 | 0.558 ± 0.093 | −2.8 ± 0.12 |
| 4 | 8 | TPGS 1000 | 0.5 | 520 ± 55.32 | 0.479 ± 0.072 | −3.08 ± 0.95 |
| 12 | 9 | TPGS 1000 | 2 | 261.2 ± 19.94 | 0.483 ± 0.043 | −1.57 ± 0.22 |
| 3 | 10 | Span 80 | 0.5 | 1054 ± 224.67 | 1.000 ± 0.000 | 1.8 ± 0. 84 |
| 1 | 11 | Tween 80 | 0.5 | 299 ± 40.40 | 0.36 ± 0.089 | −6.2 ± 1.98 |
| 6 | 12 | SDS | 1 | 189.3 ± 2.65 | 0.323 ± 0.094 | −28.2 ± 4.61 |
| N/A | N/A | N/A | N/A | 1593 ± 148.32 | 0.751 ± 0.063 | −6.86 ± 1.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witika, B.A.; Smith, V.J.; Walker, R.B. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics 2020, 12, 182. https://doi.org/10.3390/pharmaceutics12020182
Witika BA, Smith VJ, Walker RB. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics. 2020; 12(2):182. https://doi.org/10.3390/pharmaceutics12020182
Chicago/Turabian StyleWitika, Bwalya A., Vincent J. Smith, and Roderick B. Walker. 2020. "A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals" Pharmaceutics 12, no. 2: 182. https://doi.org/10.3390/pharmaceutics12020182
APA StyleWitika, B. A., Smith, V. J., & Walker, R. B. (2020). A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics, 12(2), 182. https://doi.org/10.3390/pharmaceutics12020182