Solid Dosage Forms of Dexamethasone Sodium Phosphate Intended for Pediatric Use: Formulation and Stability Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Materials
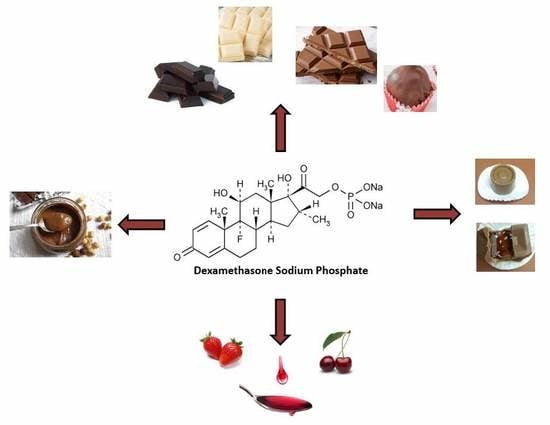
2.2. Manufacturing Process of Edible Formulations
2.2.1. Chocolate-Based Dexamethasone Sodium Phosphate (DSP) Formulations
2.2.2. Hazelnut Praline Alternative
2.2.3. Fruit Syrup/Chocolate-Based DSP Formulations
2.3. Preparation of Standard Solutions
2.4. Samples Pretreatment
2.5. Instrumentation and Method of Analysis
2.5.1. High Performance Liquid Chromatography (HPLC)
2.5.2. Dynamic Light Scattering and ζ-Potential Studies
2.5.3. X-Ray Diffraction (XRD)
2.5.4. Differential Scanning Calorimetry (DSC)
2.5.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
3.1. Sample Pre-Treatment Procedure for HPLC Analysis
3.2. Development of HPLC Conditions
3.3. HPLC Method Validation
3.3.1. Specificity
3.3.2. Linearity, Limit of Detection (LOD), Limit of Quantitation (LOQ)
3.3.3. Precision and Accuracy
3.4. Dissolution Test
3.5. In Vitro Simulation of Gastrointestinal Digestion
3.5.1. Experimental Procedure
3.5.2. DSP’s Extraction Procedure
3.5.3. Particle Size Studies
3.5.4. In Vitro Digestion
3.6. Stability Study
3.6.1. Stability of DSP During the Manufacturing Process
3.6.2. Long-Term Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cram, A.; Breitkreutz, J.; Desset-Brèthes, S.; Nunn, T.; Tuleu, C. Challenges of developing palatable oral paediatric formulations. Int. J. Pharm. 2009, 365, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Lobo, J.M.S.; et al. Patient centric pharmaceutical drug product design—The impact on medication adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef] [PubMed]
- WHO. Model List of Essential Medicines (21st List); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Santoveña-Estévez, A.; Dorta-Vera, D.; González-García, I.; Suárez-González, J.; Teigell-Pérez, N.; Fariña, J.B. Safe use of Dexamethasone in pediatrics: Design and evaluation of a novel stable oral suspension. Pharm. Technol. Hosp. Pharm. 2018, 3, 59–70. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, G.A.; Jensen, B.P.; Begg, E.J.; Bird, P.A. Determination of dexamethasone and dexamethasone sodium phosphate in human plasma and cochlear perilymph by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 17–24. [Google Scholar] [CrossRef]
- Synaridou, M.S.; Morichovitou, A.K.; Markopoulou, C.K. Innovative Pediatric Formulations: Ibuprofen in Chocolate-Coated Honey Core. J. Pharm. Innov. 2019. [CrossRef]
- Collado, M.S.; Robles, J.C.; De Zan, M.; Cámara, M.S.; Mantovani, V.E.; Goicoechea, H.C. Determination of dexamethasone and two excipients (creatinine and propylparaben) in injections by using UV-spectroscopy and multivariate calibrations. Int. J. Pharm. 2001, 229, 205–211. [Google Scholar] [CrossRef]
- Dési, E.; Kovács, Á.; Palotai, Z.; Kende, A. Analysis of dexamethasone and prednisolone residues in bovine milk using matrix solid phase dispersion-liquid chromatography with ultraviolet detection. Microchem. J. 2008, 89, 77–81. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Yang, T.; Zhang, Y. Rapid simultaneous determination of dexamethasone and betamethasone in milk by liquid chromatography tandem mass spectrometry with isotope dilution. J. Chromatogr. A 2010, 1217, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Zurbonsen, K.; Bressolle, F.; Solassol, I.; Aragon, P.J.; Culine, S.; Pinguet, F. Simultaneous determination of dexamethasone and 6β- hydroxydexamethasone in urine using solid-phase extraction and liquid chromatography: Applications to in vivo measurement of cytochrome P450 3A4 activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 804, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, B.; Guo, P.; Chen, G.; Chang, C.; Fu, Q. Facile preparation of magnetic molecularly imprinted polymers for the selective extraction and determination of dexamethasone in skincare cosmetics using HPLC. J. Sep. Sci. 2018, 41, 2441–2452. [Google Scholar] [CrossRef]
- Li, H.; Ai, L.; Fan, S.; Wang, Y.; Sun, D. Rapid determination of 18 glucocorticoids in serum using reusable on-line SPE polymeric monolithic column coupled with LC-quadrupole/orbitrap high-resolution mass spectrometer. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1065, 79–86. [Google Scholar] [CrossRef]
- Guo, P.; Chen, G.; Shu, H.; Li, P.; Yu, P.; Chang, C.; Wang, Y.; Fu, Q. Monodisperse molecularly imprinted microsphere cartridges coupled with HPLC for selective analysis of dexamethasone and hydrocortisone in cosmetics by using matrix solid-phase dispersion. Anal. Methods 2019, 11, 3687–3696. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Z.; Huang, L.; Yao, H.; Wu, X.S.; Li, S.; Lin, D. Optimization of dispersive liquid-phase microextraction based on solidified floating organic drop combined with high-performance liquid chromatography for the analysis of glucocorticoid residues in food. J. Pharm. Biomed. Anal. 2017, 138, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Beguiristain, I.; Alongi, A.; Rúbies, A.; Granados, M. Analysis of corticosteroids in samples of animal origin using QuEChERS and ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Liu, D. Detection and analysis of 17 steroid hormones by ultra-high-performance liquid chromatography-electrospray ionization mass spectrometry (UHPLC-MS) in different sex and maturity stages of Antarctic krill (Euphausia superba Dana). PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelwahab, N.S.; Ali, N.W.; Zaki, M.M.; Sharkawi, S.M.Z.; Abdelkawy, M.M. Simultaneous Determination of Thalidomide and Dexamethasone in Rat Plasma by Validated HPLC and HPTLC with Pharmacokinetic Study. J. Chromatogr. Sci. 2019, 57, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Koli, R.; Köhler, K.; Tonteri, E.; Peltonen, J.; Tikkanen, H.; Fogelholm, M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: An intervention study. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=163.130 (accessed on 1 April 2019).
- EMA (European Medicines Agency). List of Nationally Authorised Medicinal Products-Daunorubicin (PSUSA/00000936/201506); EMA (European Medicines Agency): Amsterdam, The Netherlands, 2016; Volume 44. [Google Scholar]
- Galinos. Available online: https://www.galinos.gr/web/drugs/main/drugs/soldesanil (accessed on 1 September 2019).
- Committee for Human Medicinal Products (CHMP). Questions and Answers on Propylene Glycol Used as an Excipient in Medicinal Products for Human Use. European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-propylene-glycol-used-excipient-medicinal-products-human-use_en.pdf (accessed on 9 October 2017).
- Martin, R.A. Chocolate. Adv. Food Res. 1988, 31, 211–342. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Marzec, A.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Rejch, A.; Czarkowska, K. A comparative study of thermal and textural properties of milk, white and dark chocolates. Thermochim. Acta 2019, 671, 60–69. [Google Scholar] [CrossRef]
- Fritz, J.S.; Gjerde, D.T. Ion Chromatography; Wiley-VCH Werlag GmbH & Co.: Weinheim, Germany, 2009. [Google Scholar]
- ICH Topic Q2 (R1). Validation of Analytical Procedures: Text and Methodology. ICH Harmonised Tripartite Guideline. Available online: https://doi.org/http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 1 November 2005).
- Ghulam, A. Shabir, Step-by Step Analytical Methods Validation and Protocol in the Quality System Compliance Industry, Institute of Validation and Technology. Available online: https://www.semanticscholar.org/paper/Step-by-step-analytical-methods-validation-and-in-Shabir/68718039ed36445c5e02c1100f21632f6858eff1?citationIntent=methodology#citing-papers (accessed on 3 March 2019).
- USP. Dexamethasone Tablets. Available online: http://pharmacopeia.cn/v29240/usp29nf24s0_m23340.html (accessed on 1 April 2015).
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. In Vitro digestion of infant formulations with hydrolysed and non-hydrolysed proteins from dairy and soybean. Food Funct. 2016, 7, 4908–4919. [Google Scholar] [CrossRef]
- Gould, J.; Vieira, J.; Wolf, B. Cocoa particles for food emulsion stabilisation. Food Funct. 2013, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Beato, V.M.; Sánchez, A.H.; García-García, P.; Montaño, A. Effects of selected amino acids and water-soluble vitamins on acrylamide formation in a ripe olive model system. J. Food Eng. 2014, 120, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Lipids digestibility and polyphenols release under in vitro digestion of dark, milk and white chocolate. J. Funct. Foods 2019, 52, 196–203. [Google Scholar] [CrossRef]











| DSP/Formulation (mg) | DSP/Day (mg) * | PG/Formulation (mg) | PG/Day (mg) * | Chocolate/Formulation (g) |
|---|---|---|---|---|
| 1.0 | 2.0 | 155.3 | 310.5 | 4.0 |
| 2.0 | 4.0 | 310.5 | 621.0 | 4.0 |
| Sample | Milk Chocolate | Dark Chocolate | Milk Chocolate (Stevia) | White Chocolate | Hazelnut Praline (Merenda) | Strawberry Syrup | Cherry Syrup | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked Placebo | Spiking Level (μg/mL) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) | Amount of API Found (μg/mL) | Recovery (%) |
| Sample 1 | 64.0 | 63.9 | 99.8 | 63.6 | 99.3 | 63.4 | 99.1 | 63.0 | 98.5 | 62.6 | 97.8 | 62.7 | 97.9 | 63.5 | 99.2 |
| Sample 2 | 64.0 | 64.6 | 100.9 | 64.7 | 101.1 | 64.7 | 101.1 | 63.3 | 98.9 | 63.6 | 99.3 | 63.1 | 98.6 | 63.1 | 98.6 |
| Sample 3 | 64.0 | 64.5 | 100.8 | 63.3 | 98.9 | 64.5 | 100.8 | 64.2 | 100.3 | 62.7 | 98.0 | 62.8 | 98.1 | 63.3 | 98.9 |
| Sample 1 | 32.0 | 31.7 | 99.0 | 32.3 | 100.8 | 32.4 | 101.1 | 31.7 | 99.1 | 31.2 | 97.4 | 31.3 | 97.8 | 31.5 | 98.5 |
| Sample 2 | 32.0 | 32.0 | 100.1 | 32.2 | 100.5 | 31.6 | 98.6 | 32.3 | 101.0 | 32.3 | 100.9 | 31.3 | 97.9 | 31.7 | 99.2 |
| Sample 3 | 32.0 | 32.3 | 101.1 | 31.7 | 99.2 | 31.7 | 99.2 | 32.4 | 101.4 | 31.8 | 99.4 | 32.0 | 99.9 | 31.6 | 98.9 |
| Sample 1 | 8.0 | 7.9 | 98.6 | 7.8 | 98.1 | 7.9 | 99.3 | 7.8 | 98.0 | 8.1 | 100.8 | 8.0 | 99.4 | 7.9 | 98.6 |
| Sample 2 | 8.0 | 8.0 | 100.2 | 7.9 | 99.3 | 8.0 | 99.8 | 7.9 | 98.4 | 7.9 | 98.9 | 7.9 | 98.6 | 8.0 | 99.6 |
| Sample 3 | 8.0 | 7.9 | 99.0 | 8.0 | 99.4 | 8.1 | 100.9 | 7.9 | 99.1 | 8.0 | 99.5 | 7.9 | 98.9 | 7.9 | 98.9 |
| Mean | 99.9 | Mean | 99.6 | Mean | 100.0 | Mean | 99.4 | Mean | 99.1 | Mean | 98.6 | Mean | 98.9 | ||
| %RSD | 0.9 | %RSD | 0.9 | %RSD | 0.9 | %RSD | 1.1 | %RSD | 1.2 | %RSD | 0.7 | %RSD | 0.3 | ||
| Type of Chocolate | Hardness * (kp) | Friability ** | Disintegration Time *** (min) |
|---|---|---|---|
| Milk chocolate | 8.8 | Loss of mass = 0.2% | - |
| Milk chocolate + DSP | 5.9 | Loss of mass = 0.3% | 20 |
| Milk chocolate (stevia) | 8.5 | Loss of mass = 0.2% | - |
| Milk chocolate (stevia) + DSP | 5.7 | Loss of mass = 0.3% | 22 |
| Dark chocolate | 9.2 | Loss of mass = 0.1% | - |
| Dark chocolate + DSP | 6.3 | Loss of mass = 0.2% | 36 |
| White chocolate | 7.6 | Loss of mass = 0.2% | - |
| White chocolate + DSP | 4.0 | Loss of mass = 0.5% | 24 |
| Formulation | ζ-Potential ± Standard Deviation (mV) in Distilled Water | ζ-Potential ± Standard Deviation (mV) in SGF |
|---|---|---|
| Milk Chocolate | −13.5 ± 0.0 | −8.2 ± 17.1 |
| Milk Chocolate + DSP | −11.4 ± 9.37 | −9.0 ± 27.7 |
| White Chocolate | −14.0 ± 21.6 | −9.9 ± 21.8 |
| White Chocolate + DSP | −13.6 ± 11.2 | −9.8 ± 8.6 |
| Dark Chocolate | −15.3 ± 13.7 | −12.7 ± 14.3 |
| Dark Chocolate + DSP | −14.9 ± 9.3 | −11.7 ± 14.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Synaridou, M.S.; Andriotis, E.G.; Zacharis, C.K.; Fatouros, D.G.; Markopoulou, C.K. Solid Dosage Forms of Dexamethasone Sodium Phosphate Intended for Pediatric Use: Formulation and Stability Studies. Pharmaceutics 2020, 12, 354. https://doi.org/10.3390/pharmaceutics12040354
Synaridou MS, Andriotis EG, Zacharis CK, Fatouros DG, Markopoulou CK. Solid Dosage Forms of Dexamethasone Sodium Phosphate Intended for Pediatric Use: Formulation and Stability Studies. Pharmaceutics. 2020; 12(4):354. https://doi.org/10.3390/pharmaceutics12040354
Chicago/Turabian StyleSynaridou, Maria S., Eleftherios G. Andriotis, Constantinos K. Zacharis, Dimitrios G. Fatouros, and Catherine K. Markopoulou. 2020. "Solid Dosage Forms of Dexamethasone Sodium Phosphate Intended for Pediatric Use: Formulation and Stability Studies" Pharmaceutics 12, no. 4: 354. https://doi.org/10.3390/pharmaceutics12040354
APA StyleSynaridou, M. S., Andriotis, E. G., Zacharis, C. K., Fatouros, D. G., & Markopoulou, C. K. (2020). Solid Dosage Forms of Dexamethasone Sodium Phosphate Intended for Pediatric Use: Formulation and Stability Studies. Pharmaceutics, 12(4), 354. https://doi.org/10.3390/pharmaceutics12040354