Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions
Abstract
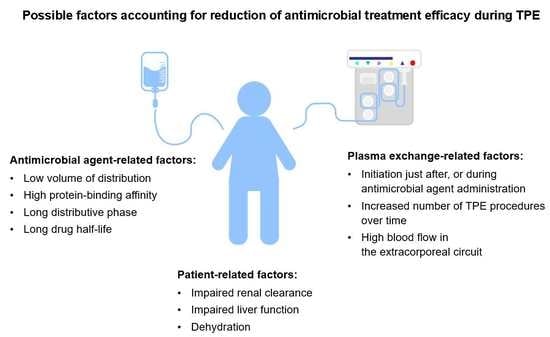
:1. Introduction
2. Clinical Use
- Myasthenia gravis—removal of anti-AChR and anti-MuSK antibodies;
- Thrombocytopenic purpura—removal of anti-ADAMTS13 IgG autoantibodies;
- Guillian–Barré Syndrome—removal of various autoantibodies against gangliosides including GM1, GD1a, GalNAc-GD1a etc.;
- Wilson’s disease (fulminant)—removal of copper.
- Lambert–Eaton myasthenic syndrome—removal of autoantibodies against the voltage-gated calcium channel (VGCC);
- Systemic lupus erythematosus (severe);
- Myeloma cast nephropathy—removal of light chains (Bence–Jones protein);
- Mushroom poisoning.
- Autoimmune hemolytic anemia—removal of IgG hemolysins;
- Hypertrigliceridemic pancreatitis—lowering triglyceride levels, reduction of inflammatory cytokines, and potential replacement of deficient LpL or apolipoproteins when plasma is used as the replacement fluid;
- Immune thrombocytopenia—removal of autoantibodies against platelet surface antigens, primarily GPIIb/IIIa and/or GPIb/IX;
- Immunoglobulin A nephropathy—removal of pathological IgA and related immune complexes;
- Sepsis with multi-organ failure.
- Psoriasis
- Systemic Amyloidosis
- Amyotrophic Lateral Sclerosis
- Polymyositis/dermatomyositis
3. Procedure
4. Basics of Pharmacokinetics During TPE
5. Antimicrobials During TPE
5.1. Beta-Lactams
5.2. Penicilines
5.2.1. Ampicillin
5.2.2. Piperacillin
5.3. Cephalosporins
5.3.1. Ceftriaxone
5.3.2. Ceftazidime
5.3.3. Cefepime
5.4. Monobactams
Aztreonam
5.5. Carbapenems
5.5.1. Imipenem
5.5.2. Meropenem
5.6. Glycopeptides
5.6.1. Vancomycin
5.6.2. Teicoplanin
5.7. Aminoglycosides
Tobramycin
5.8. Fluoroquinolones
5.9. Macrolides
5.10. Colistin
5.11. Antivirals
5.11.1. Acyclovir
5.11.2. Oseltamivir
5.12. Antifungals
5.12.1. Amphotericin B (liposomal)
5.12.2. Voriconazole
5.13. Other Antimicrobials
6. Conclusions
Funding
Conflicts of Interest
References
- Clark, W.F.; Huang, S.S. Introduction to therapeutic plasma exchange. Transfus. Apher. Sci. 2019, 58, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Vatazin, A.V.; Zulkarnaev, A.B. The impact of therapeutic plasma exchange and double filtration plasmapheresis on hemostasis in renal transplant recipients. Ter. Arkh. 2018, 90, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kutnik, P.; Szczukocka, M.; Borys, M.; Czuczwar, M. Procalcitonin dynamics, lactates, and haemoglobin serum levels might be a useful predictive tool of mortality in patients undergoing veno-venous extracorporeal oxygenation membrane support. Single centre experience. Anaesthesiol. Intensive Ther. 2019, 51, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the use of therapeutic apheresis in clinical practice—Evidence-based approach from the writing committee of the american society for apheresis: The eighth special issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef]
- Ponikvar, R. Blood purification in the intensive care unit. Nephrol. Dial. Transplant. 2003, 18, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, A.; Parquet, N.; Galicier, L.; Boutboul, D.; Bertinchamp, R.; Malphettes, M.; Dumas, G.; Mariotte, E.; Peraldi, M.-N.; Souppart, V.; et al. Plasma exchange in the intensive care unit: Technical aspects and complications. J. Clin. Apher. 2017, 32, 405–412. [Google Scholar] [CrossRef]
- Mörtzell Henriksson, M.; Newman, E.; Witt, V.; Derfler, K.; Leitner, G.; Eloot, S.; Dhondt, A.; Deeren, D.; Rock, G.; Ptak, J.; et al. Adverse events in apheresis: An update of the WAA registry data. Transfus. Apher. Sci. 2016, 54, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Serednicki, W.; Cicio, M. Plasmapheresis as a treatment method for patients admitted to Critical Care Unit. Anest Ratow. 2019, 13, 270–279. [Google Scholar]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Betz, C.; Buettner, S.; Geiger, H.; Jung, O. Regional citrate anticoagulation in therapeutic plasma exchange with fresh frozen plasma—A modified protocol. Int. J. Artif. Organs. 2013, 36, 803–811. [Google Scholar] [CrossRef]
- Scaravilli, V.; Di Girolamo, L.; Scotti, E.; Busana, M.; Biancolilli, O.; Leonardi, P.; Carlin, A.; Lonati, C.; Panigada, M.; Pesenti, A.; et al. Effects of sodium citrate, citric acid and lactic acid on human blood coagulation. Perfusion 2018, 33, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Linenberger, M.L.; Price, T.H. Use of cellular and plasma apheresis in the critically ill patient: Part 1: Technical and physiological considerations. J. Intensive Care Med. 2005, 20, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Rimmelé, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, B.C. Therapeutic apheresis: Use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract. Res. Clin. Haematol. 2006, 19, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.E. Plasma exchange: Why we do what we do. J. Clin. Apher. 2002, 17, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Sketris, I.S.; Parker, W.A.; Jones, J.V. Plasmapheresis: Its effect on toxic agents and drugs. Plasma Ther. Transfus. Technol. 1984, 5, 305–361. [Google Scholar]
- Ibrahim, R.B.; Balogun, R.A. Medications in patients treated with therapeutic plasma exchange: Prescription dosage, timing, and drug overdose. Semin. Dial. 2012, 25, 176–189. [Google Scholar] [CrossRef]
- Charlton, M.; Thompson, J.P. Pharmacokinetics in sepsis. BJA Educ. 2019, 19, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Power, B.M.; Forbes, A.M.; van Heerden, P.V.; Ilett, K.F. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharm. 1998, 34, 25–56. [Google Scholar] [CrossRef]
- McNamara, P.J.; Gibaldi, M.; Stoeckel, K. Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding II. Physiological significance. Eur. J. Clin. Pharmacol. 1983, 25, 407–412. [Google Scholar] [CrossRef]
- Khan, E.; Huggan, P.; Celi, L.; MacGinley, R.; Schollum, J.; Walker, R. Sustained low-efficiency dialysis with filtration (SLEDD-f) in the management of acute sodium valproate intoxication. Hemodial. Int. 2008, 12, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Roberts, D.M.; Hoffman, R.S.; Ouellet, G.; Roy, L.; Decker, B.S.; Bouchard, J. A stepwise approach for the management of poisoning with extracorporeal treatments. Semin. Dial. 2014, 27, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.B.; Balogun, R.A. Medications and therapeutic apheresis procedures: Are we doing our best? J. Clin. Apher. 2013, 28, 73–77. [Google Scholar] [CrossRef]
- Ibrahim, R.B.; Liu, C.; Cronin, S.M.; Murphy, B.C.; Cha, R.; Swerdlow, P.; Edwards, D.J. Drug removal by plasmapheresis: An evidence-based review. Pharmacotherapy 2007, 27, 1529–1549. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, F.; Petitjean, O.; Tod, M.; Guillevin, L. Clinical pharmacokinetics during plasma exchange. Therapie 2000, 55, 269–275. [Google Scholar]
- Fauvelle, F.; Leon, A.; Niakate, M.T.; Petitjean, O.; Guillevin, L. Diclofenac, paracetamol, and vidarabine removal during plasma exchange in polyarteritis nodosa patients. Biopharm. Drug Dispos. 1991, 12, 411–424. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2012-07-11_Ceftriaxone_Actavis_2g_ChPL.pdf (accessed on 15 March 2020).
- Summary of Product Characteristics. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050640s017lbl.pdf (accessed on 15 March 2020).
- Prince, A.S.; Kliegman, R.; Phaneuf, D.; Neu, H.C. The effect of exchange-transfusion on the blood levels of ampicillin and gentamicin in neonates. Infection 1981, 9, 2–6. [Google Scholar] [CrossRef]
- Roberts, J.A.; Roberts, M.S.; Robertson, T.A.; Cross, S.E.; Lipman, J. A novel way to investigate the effects of plasma exchange on antibiotic levels: Use of microdialysis. Int. J. Antimicrob. Agents. 2008, 31, 240–244. [Google Scholar] [CrossRef]
- Kintzel, P.E.; Eastlund, T.; Calis, K.A. Extracorporeal removal of antimicrobials during plasmapheresis. J. Clin. Apher. 2003, 18, 194–205. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Penicillin g, CID = 5904. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/Penicillin-g (accessed on 17 March 2020).
- Ticarcillin: Prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050658s023,050590s058,050590s059lbl.pdf (accessed on 15 March 2020).
- Fauvelle, F.; Lortholary, O.; Tod, M.; Guillevin, L.; Louchahi, M.; Léon, A.; Petitjean, O. Pharmacokinetics of ceftriaxone during plasma exchange in polyarteritis nodosa patients. Antimicrob. Agents Chemother. 1994, 38, 1519–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakken, J.S.; Cavalieri, S.J.; Gangeness, D.; Kubat, T.; Pollack, J.R. Influence of therapeutic plasmapheresis on elimination of ceftriaxone. Antimicrob. Agents Chemother. 1993, 37, 1171–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceftazidime: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2012-1219_Fortum_1g_SmPC_01_08_2012_clean.pdf (accessed on 14 March 2020).
- Benoni, G.; Arosio, E.; Raimondi, M.G.; Pancera, P.; Lechi, A.; Velo, G.P. Pharmacokinetics of ceftazidime and ceftriaxone and their penetration into the ascitic fluid. J. Antimicrob. Chemother. 1985, 16, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Nye, K.J.; Shi, Y.G.; Andrews, J.M.; Wise, R. Pharmacokinetics and tissue penetration of cefepime. J. Antimicrob. Chemother. 1989, 24, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.B.; Liu, C.Y.; Cronin, S.M.; Murphy, B.C.; Cha, R.; Swerdlow, P.; Avila, T.; Smith, S.T.; Lewis, R.A.; Edwards, D.J. Influence of plasma exchange on the disposition of the fourth generation cephalosporin cefepime. J. Oncol. Pharm. Pract. 2009, 15, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cefazolin: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2013-02-07_TarfazolinChPLRe-rej310113.pdf (accessed on 14 March 2020).
- Cefuroxime: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2012-09-21_Cefuroxim-MIP_1500_ChPL.pdf (accessed on 14 March 2020).
- Cefotaxime: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2013-04-24_SmPC_Biotaksym_2013_04P.pdf (accessed on 14 March 2020).
- Ceftaroline: Summary of Product Characteristics. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/200327s001lbl.pdf (accessed on 17 March 2020).
- Mattie, H. Clinical pharmacokinetics of aztreonam. Clin. Pharm. 1988, 14, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Imipenem: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=6675-c (accessed on 17 March 2020).
- Mouton, J.W.; van den Anker, J.N. Meropenem clinical pharmacokinetics. Clin. Pharm. 1995, 28, 275–286. [Google Scholar] [CrossRef]
- Vancomycin: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2010-12-28_20101214_vancomycin_billev_1000_spc_(ib_4)-ur-clean.pdf (accessed on 17 March 2020).
- Boeckh, M.; Lode, H.; Borner, K.; Hoffken, G.; Wagner, J.; Koeppe, P. Pharmacokinetics and serum bacteriocidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob. Agent. Chemother. 1988, 32, 92–95. [Google Scholar] [CrossRef] [Green Version]
- McClellan, S.D.; Whitaker, C.H.; Friedberg, R.C. Removal of vancomycin during plasmapheresis. Ann. Pharm. 1997, 31, 1132–1136. [Google Scholar] [CrossRef]
- Brophy, D.F.; Mueller, B.A. Vancomycin removal by plasmapheresis. Ann. Pharm. 1996, 30, 1038. [Google Scholar] [CrossRef]
- Osman, B.A.; Lew, S.Q. Vancomycin removal by plasmapheresis. Pharm. Toxicol. 1997, 81, 245–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alet, P.; Lortholary, O.; Fauvelle, F.; Tod, M.; Genereau, T.; Louchahi, M.; Leon, A.; Guillevin, L.; Petitjean, L. Pharmacokinetics of teicoplanin during plasma exchange. Clin. Microbiol. Infect. 1999, 5, 213–218. [Google Scholar] [PubMed] [Green Version]
- Vogelman, B.; Gudmundsson, S.; Leggett, J.; Turnidge, J.; Ebert, S.; Craig, W.A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 1988, 158, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. North. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.D.; Lietman, P.S.; Smith, C.R. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 1987, 155, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Aarons, L.; Vozeh, S.; Wenk, M.; Weiss, P.; Follath, F. Population pharmacokinetics of tobramycin. Br. J. Clin. Pharmacol. 1989, 28, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Pechere, J.C.; Roy, B.; Dugal, R. Distribution and elimination kinetics of intravenously and intramuscularly administered tobramycin in man. Int. J. Clin. Pharmacol. 1976, 14, 313–318. [Google Scholar]
- Oullette, S.M.; Visconti, J.A.; Kennedy, M.S. A pharmacokinetic evaluation of the effect of plasma exchange on tobramycin disposition. Clin. Exp. Dial. Apher. 1983, 7, 225–233. [Google Scholar] [CrossRef]
- Kale-Pradhan, P.B.; Dehoorne-Smith, M.L.; Jaworski, D.A.; Hare, B.L.; Provenzano, R.; Higgins, M.J. Evaluation of plasmapheresis on the removal of tobramycin. Pharmacotherapy 1995, 15, 673–676. [Google Scholar] [CrossRef]
- Amikacin: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=1035-c (accessed on 14 March 2020).
- Gentamycin: Summary of Product Characteristics. Available online: http://leki.urpl.gov.pl/files/43_Gentamicin_KRKA_roztw_do_wstrzyk_inf_40mg_ml.pdf (accessed on 14 March 2020).
- Streptomycin: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=6407-c (accessed on 14 March 2020).
- Ciprofloxacin: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2012-12-03_Ciprofloxacin_Kabi_ChPL.pdf (accessed on 14 March 2020).
- Levofloxacin: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/2013-01-28_Levoxa_roztw_infuz_ChPL.pdf (accessed on 14 March 2020).
- Azithromycin: Summary of Product Characteristics. Available online: http://leki.urpl.gov.pl/files/25_AzitroLEK500_tab_powl_500.pdf (accessed on 15 March 2020). (In Polish)
- Womble, A.Y.; Giguère, S.; Lee, E.A.; Vickroy, T.W. Pharmacokinetics of clarithromycin and concentrations in body fluids and bronchoalveolar cells of foals. Am. J. Vet. Res. 2006, 67, 1681–1686. [Google Scholar] [CrossRef]
- Couet, W.; Grégoire, N.; Marchand, S.; Mimoz, O. Colistin pharmacokinetics: The fog is lifting. Clin. Microbiol. Infect. 2012, 18, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Smeaton, T.C.; Coulthard, K. Pharmacokinetics of Colistin Methanesulphonate and Colistin in Rats Following an Intravenous Dose of Colistin Methanesulphonate. J. Antimicrob. Chemother. 2004, 53, 837–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acyclovir: Summary of Product Characteristics. Available online: http://chpl.com.pl/data_files/20130613_aciclovir_jelfa_inj_spc_clean_2013_05_24.pdf (accessed on 14 March 2020).
- Blum, M.R.; Liao, S.H.T.; de Miranda, P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am. J. Med. 1982, 73, 186–192. [Google Scholar] [CrossRef]
- Spector, S.A.; Connor, J.D.; Hintz, M.; Quinn, R.P.; Blum, M.R.; Keeney, R.E. Single-dose pharmacokinetics of acyclovir. Antimicrob. Agent. Chemother. 1981, 19, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Miranda, P.; Good, S.S.; Laskin, O.L.; Krasny, H.C.; Connor, J.D.; Lietman, P.S. Disposition of intravenous radioactive acyclovir. Clin. Pharmacol. Ther. 1981, 30, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Chavanet, P.Y.; Bailly, F.; Mousson, C.; Waldner-Combernoux, A.; Lokiec, F.; Rebibou, J.M.; Chalopin, J.M.; Portier, H. Acyclovir pharmacokinetics in plasmapheresis. J. Clin. Apher. 1990, 5, 68–69. [Google Scholar]
- Oseltamivir: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=36011-c (accessed on 16 March 2020). (In Polish)
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Lew, S.Q. Amphotericin B removal by plasma exchange. J. Clin. Pharm. Ther. 2009, 34, 115–117. [Google Scholar] [CrossRef]
- Spriet, I.; Brüggemann, R.J.; Annaert, P.; Meersseman, P.; Van Wijngaerden, E.; Lagrou, K.; Willems, L. Pharmacokinetic profile of voriconazole in a critically ill patient on therapeutic plasma exchange. Ther. Drug Monit. 2013, 35, 141–143. [Google Scholar] [CrossRef]
- Metronidazole: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=4355-c (accessed on 16 March 2020).
- Clindamycin: Summary of Product Characteristics. Available online: http://leki.urpl.gov.pl/files/25_Klimicin_roztw_do_wstrzyk_i_inf_300_600.pdf (accessed on 16 March 2020).
- Sulfamethoxazole: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=1081-c (accessed on 16 March 2020).
- Linezolid: Summary of Product Characteristics. Available online: https://pub.rejestrymedyczne.csioz.gov.pl/Pobieranie.ashx?type=32789-c (accessed on 16 March 2020).




| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Ampicillin [30] | 0.2–0.3 | 20% | N/A | 60–80 | moderate |
| Amoxicillin [32] | 0.21 | 18 | N/A | 68 | moderate/insignificant |
| Penicillin G [33] | 0.53–0.67 | 45-68 | N/A | 60–90 | insignificant |
| Ticarcillin [34] | 0.17–0.23 | 45–65 | N/A | 60–70 | moderate/insignificant |
| Piperacillin [28] | 0.24 | 30% | N/A | 68 | insignificant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Cefazolin (1st gen.) [41] | 0.19 | 88 | N/A | 80 | significant |
| Cefuroxime (2nd gen.) [42] | 0.2 | 33–50 | N/A | 96 | moderate/insignificant |
| Ceftazidime [37] | 0.23 | 10 | 16–31 | 99 | moderate/insignificant |
| Ceftriaxone [28] | 0.13 | 95 | 14–42 | 50–60 | significant |
| Cefotaxime (3rd gen) [43] | 0.23 | 30 | N/A | 50 | insignificant |
| Cefepime [39] | 0.32 | 20 | 18 | 85 | insignificant |
| Ceftaroline (5th gen) [44] | 0.37 | 20 | N/A | 88 | insignificant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction Of TPE Influence |
|---|---|---|---|---|---|
| Amikacin [61] | 0.27 | 4 | N/A | >90 | insignificant |
| Gentamicin [62] | 0.25 | 0–30 | 21–41 | >90 | insignificant |
| Streptomycin [63] | 0.26 | 35 | N/A | >90 | insignificant |
| Tobramycin [57,58] | 0.33 | <10 | 6–20 | 90 | insignificant |
| Kanamycin [32] | 0.19 | 0–3 | N/A | >90 | moderate/insignificant |
| Netilmycin [32] | 0.16–0.34 | 0–30 | N/A | >80 | moderate/insignificant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Ciprofloxacin [64] | 2–3 | 20–30 | N/A | ~70 | insignificant |
| Levofloxacin [65] | 1.1 | 30–40 | 27 | >85 | insignificant |
| Moxifloxacin [32] | 2 | 30–40 | N/A | ~40 | insignificant |
| Ofloxacin [32] | 1.8 | 25 | N/A | ~90 | insignificant |
| Norfloxacin [32] | 0.36–0.5 | 10–15 | N/A | ~65 | insignificant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Azithromycin [66] | 0.44 | 12–52 | N/A | 12–20 | insignificant |
| Clarithromycin [67] | 2.6 | 70 | N/A | 15–30 | insignificant |
| Erythromycin [32] | 0.78 | 84 | N/A | 2.5 | moderate/insignificant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Amphotericin B [73] | 0.1–0.2 | 95–99 | N/A | N/A | significant |
| Ketoconazole [32] | 2.4 | 99 | N/A | 13 | moderate/significant |
| Fluconazole [32] | 0.6 | 11 | N/A | 80 | insignificant |
| Voriconazole [32] | 4.5 | 58 | N/A | 94 | insignificant |
| Terbinafine [32] | >29 | 99 | N/A | N/A | moderate/insignificant |
| Caspofungin [32] | 0.3–2 | 97 | N/A | 41 | moderate/significant |
| Antibiotic | Distribution Volume [L kg−1] | Protein Binding [%] | Distribution Half-Life [min] | Renal Clearance [%] | Prediction of TPE Influence |
|---|---|---|---|---|---|
| Metronidazole [79] | 0.25–0.85 | <20 | N/A | 60–80 | insignificant |
| Clindamycin [80] | 1.1 | 60–94 | N/A | ~33 | moderate/insignificant |
| Sulfamethoxazole [81] | 0.43 | 70 | N/A | 84.5 | moderate/insignificant |
| Trimethoprim [81] | 0.7–1.5 | 50 | N/A | 66.8 | insignificant |
| Linezolid [82] | 0.57–0.86 | 31 | N/A | 35 | insignificant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzych, Ł.J.; Czok, M.; Putowski, Z. Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions. Pharmaceutics 2020, 12, 395. https://doi.org/10.3390/pharmaceutics12050395
Krzych ŁJ, Czok M, Putowski Z. Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions. Pharmaceutics. 2020; 12(5):395. https://doi.org/10.3390/pharmaceutics12050395
Chicago/Turabian StyleKrzych, Łukasz J., Marcelina Czok, and Zbigniew Putowski. 2020. "Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions" Pharmaceutics 12, no. 5: 395. https://doi.org/10.3390/pharmaceutics12050395
APA StyleKrzych, Ł. J., Czok, M., & Putowski, Z. (2020). Is Antimicrobial Treatment Effective During Therapeutic Plasma Exchange? Investigating the Role of Possible Interactions. Pharmaceutics, 12(5), 395. https://doi.org/10.3390/pharmaceutics12050395