Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials:
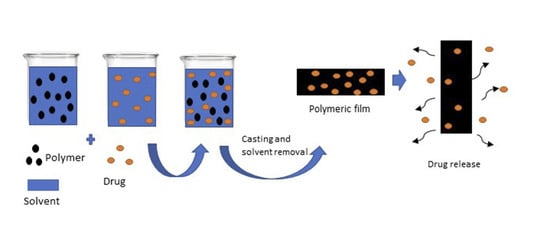
2.2. Preparation of DTX-Loaded PSi Films
2.3. Preparation of DTX-Loaded PEVA and PU Films
2.4. Photoacoustic Fourier-Transform Infrared (PA-FTIR) Spectroscopy of Films
2.5. X-Ray Powder Diffraction (XRD) of Films
2.6. Scanning Electron Microscopy (SEM) of Films
2.7. Thermal Analysis of Films
2.8. Mechanical Properties of the Films
2.9. HPLC Analysis of DTX
2.10. Determination of Drug-Loading in Films
2.11. Determination of DTX Solubility
2.12. In Vitro Drug Release
3. Results
3.1. Preparation of DTX-Loaded Films
3.2. PA-FTIR Spectrophotometry of DTX-Loaded Films
3.3. XRD of DTX-Loaded Films
3.4. Surface Topography of the DTX-Loaded Films
3.5. Thermal Analysis of DTX-Loaded Films
3.6. Mechanical Properties
3.7. Determination of DTX Loading
3.8. In Vitro Drug Release
3.9. Drug Release Mechanism
3.10. Degradation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, S.W.; Kim, T.I.; Lee, J.-H.; Lee, B.-I.; Keum, B.; Cheung, D.Y.; Yang, C.H.; The Stent Study Group of the Korean Society of Gastrointestinal Endoscopy. Evidence-based recommendations on colorectal stenting: A report from the stent study group of the korean society of gastrointestinal endoscopy. Clin. Endosc. 2013, 46, 355–367. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.J. Stents for colorectal obstruction: Past, present, and future. World J. Gastroenterol. 2016, 22, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Fang, J.; Wong, R.; Wills, J.; Hilden, K. Placement of polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest. Endosc. 2009, 70, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Ailawadhi, S.; Yang, G.Y.; Nwogu, C.; Schiff, M.D.; Nava, H.R. Palliation of malignant dysphagia in esophageal cancer: A literature-based review. J. Supportive Oncol. 2006, 4, 365–373, 379. [Google Scholar]
- Sharma, P.; Kozarek, R.; The Practice Parameters Committee of the American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am. J. Gastroenterol. 2010, 105, 258–273, quiz 274. [Google Scholar] [CrossRef]
- Madhusudhan, C.; Saluja, S.S.; Pal, S.; Ahuja, V.; Saran, P.; Dash, N.R.; Sahni, P.; Chattopadhyay, T.K. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: Impact on quality of life. Dis. Esophagus 2009, 22, 331–336. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, S.; Wang, Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing eva layer and one drug-free protective layer: In vitro release, permeation and mechanical properties. J. Control. Release 2007, 118, 318–324. [Google Scholar] [CrossRef]
- Rong, H.-J.; Chen, W.-L.; Guo, S.-R.; Lei, L.; Shen, Y.-Y. Pcl films incorporated with paclitaxel/5-fluorouracil: Effects of formulation and spacial architecture on drug release. Int. J. Pharm. 2012, 427, 242–251. [Google Scholar] [CrossRef]
- Lei, L.; Liu, X.; Guo, S.; Tang, M.; Cheng, L.; Tian, L. 5-fluorouracil-loaded multilayered films for drug controlled releasing stent application: Drug release, microstructure, and ex vivo permeation behaviors. J. Control. Release 2010, 146, 45–53. [Google Scholar] [CrossRef]
- Guo, Q.; Knight, P.T.; Mather, P.T. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. J. Control. Release 2009, 137, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ako, J.; Bonneau, H.N.; Honda, Y.; Fitzgerald, P.J. Design criteria for the ideal drug-eluting stent. Am. J. Cardiol. 2007, 100, S3–S9. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Park, S. Local delivery of antiproliferative agents via stents. Polymers 2014, 6, 755–775. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.; Kichenadasse, G.; Choudhury, N.R.; Butler, R.; Garg, S. Non-vascular drug eluting stents as localized controlled drug delivery platform: Preclinical and clinical experience. J. Control. Release 2013, 172, 105–117. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Blencowe, A.; Albrecht, H.; Song, Y.; Garg, S. Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J. Control. Release 2019, 308, 209–231. [Google Scholar] [CrossRef]
- Baudis, S.; Nehl, F.; Ligon, S.C.; Nigisch, A.; Bergmeister, H.; Bernhard, D.; Stampfl, J.; Liska, R. Elastomeric degradable biomaterials by photopolymerization-based cad-cam for vascular tissue engineering. Biomed. Mater. 2011, 6, 055003. [Google Scholar] [CrossRef]
- Giladi, N.; Boroojerdi, B.; Korczyn, A.D.; Burn, D.J.; Clarke, C.E.; Schapira, A.H.V.; SP513 investigators. Rotigotine transdermal patch in early parkinson’s disease: A randomized, double-blind, controlled study versus placebo and ropinirole. Mov. Disord. 2007, 22, 2398–2404. [Google Scholar] [CrossRef]
- MASHAK, A.; RAHIMI, A.J.I.P.J. Silicone polymers in controlled drug delivery systems: A review. Iran. Polym. J. 2009, 18, 279–295. [Google Scholar]
- Rider, J.A.; Moeller, H.C. Use of silicone in the treatment of intestinal gas and bloating. JAMA 1960, 174, 2052–2054. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O. Sustained release drug delivery applications of polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.M.; Pearce, S.M.; Poursaid, A.E.; Aliyar, H.A.; Tresco, P.A.; Mitchnik, M.A.; Kiser, P.F. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J. Pharm. Sci. 2008, 97, 4228–4239. [Google Scholar] [CrossRef]
- Bucky, L.P.; Ehrlich, H.P.; Sohoni, S.; May, J.W. The capsule quality of saline-filled smooth silicone, textured silicone, and polyurethane implants in rabbits: A long-term study. Plast. Reconstr. Surg. 1994, 93, 1123–1131, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Ekin, A.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; VanderWal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. A preliminary study on the properties and fouling-release performance of siloxane–polyurethane coatings prepared from poly(dimethylsiloxane) (PDMS) macromers. Biofouling 2010, 26, 961–972. [Google Scholar] [CrossRef]
- Jansen, B.; Goodman, L.P.; Ruiten, D. Bacterial adherence to hydrophilic polymer–coated polyurethane stents. Gastrointest. Endosc. 1993, 39, 670–673. [Google Scholar] [CrossRef]
- Claeys, B.; Vervaeck, A.; Hillewaere, X.K.D.; Possemiers, S.; Hansen, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Eur. J. Pharm. Biopharm. 2015, 90, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3d printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef]
- Novák, A.; de la Loge, C.; Abetz, L.; van der Meulen, E.A. The combined contraceptive vaginal ring, NuvaRing®: An international study of user acceptability. Contraception 2003, 67, 187–194. [Google Scholar] [CrossRef]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Le, J.; Tsourounis, C. Implanon: A critical review. Ann. Pharmacother. 2001, 35, 329–336. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Kwak, T.W.; Lee, H.L.; Song, Y.H.; Kim, C.; Kim, J.; Seo, S.-J.; Jeong, Y.-I.; Kang, D.H. Vorinostat-eluting poly(DL-lactide-co-glycolide) nanofiber-coated stent for inhibition of cholangiocarcinoma cells. Int. J. Nanomed. 2017, 12, 7669–7680. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Lee, D.H.; Lee, D.K.; Na, K. Nonvascular drug-eluting stent coated with sodium caprate-incorporated polyurethane for the efficient penetration of paclitaxel into tumor tissue. J. Biomater. Appl. 2014, 29, 1133–1144. [Google Scholar] [CrossRef]
- Seo, E.H.; Na, K. Polyurethane membrane with porous surface for controlled drug release in drug eluting stent. Biomater. Res. 2014, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Kim, M.; Kim, M.-k.; Lee, H.; Lee, D.K.; Lee, D.-H.; Yang, S.-G. Paclitaxel-eluting nanofiber-covered self-expanding nonvascular stent for palliative chemotherapy of gastrointestinal cancer and its related stenosis. Biomed. Microdevices 2014, 16, 897–904. [Google Scholar] [CrossRef]
- Yuk, S.H.; Oh, K.S.; Park, J.; Kim, S.-J.; Kim, J.H.; Kwon, I.K. Paclitaxel-loaded poly(lactide-co-glycolide)/poly(ethylene vinyl acetate) composite for stent coating by ultrasonic atomizing spray. Sci. Technol. Adv. Mater. 2012, 13, 025005. [Google Scholar] [CrossRef]
- Lee, J.W.; Yang, S.-G.; Na, K. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int. J. Pharm. 2012, 427, 276–283. [Google Scholar] [CrossRef]
- Lee, S.S.; Shin, J.H.; Han, J.M.; Cho, C.H.; Kim, M.-H.; Lee, S.-K.; Kim, J.-H.; Kim, K.-R.; Shin, K.M.; Won, H.-Y.; et al. Histologic influence of paclitaxel-eluting covered metallic stents in a canine biliary model. Gastrointest. Endosc. 2009, 69, 1140–1147. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, H.S.; Kim, K.-S.; Lee, W.J.; Kim, H.K.; Won, Y.H.; Byun, Y.R.; Kim, M.Y.; Baik, S.K.; Kwon, S.O. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest. Endosc. 2005, 61, 296–301. [Google Scholar] [CrossRef]
- Jang, S.I.; Kim, J.-H.; You, J.W.; Rhee, K.; Lee, S.J.; Kim, H.G.; Han, J.; Shin, I.H.; Park, S.-H.; Lee, D.K. Efficacy of a metallic stent covered with a paclitaxel-incorporated membrane versus a covered metal stent for malignant biliary obstruction: A prospective comparative study. Dig. Dis. Sci. 2013, 58, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, J.W.; Kim, H.S.; Baik, S.K.; Oh, S.J.; Lee, S.J.; Kim, H.G.; Lee, D.H.; Won, Y.H.; Lee, D.K. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: Multicenter pilot study. Gastrointest. Endosc. 2007, 66, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Lee, S.S.; Yun, S.C.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.-H. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: A prospective comparative pilot study. Gastroint. Endosc. 2011, 73, 727–733. [Google Scholar] [CrossRef]
- Manifold, D.K.; Maynard, N.D.; Cowling, M.; Machan, L.; Mason, R.C.; Adam, A. Taxol coated stents in oesophageal adenocarcinoma. Gastroenterology 1998, 114, A27. [Google Scholar] [CrossRef]
- Dai, Z.; Zhou, D.; Hu, J.; Zhang, L.E.I.; Lin, Y.; Zhang, J.; Li, F.; Liu, P.; Li, H.U.A.; Cao, F. Clinical application of iodine-eluting stent in patients with advanced esophageal cancer. Oncol. Lett. 2013, 6, 713. [Google Scholar] [CrossRef] [Green Version]
- Heath, E.I.; Urba, S.; Marshall, J.; Piantadosi, S.; Forastiere, A.A. Phase II trial of docetaxel chemotherapy in patients with incurable adenocarcinoma of the esophagus. Investig. New Drugs 2002, 20, 95–99. [Google Scholar] [CrossRef]
- Shakuto, S.; Fujita, F.; Fujita, M. Antitumor effect of docetaxel against human esophagus tumor cell lines and tumor xenografts in nude mice. Gan Kagaku Ryoho Cancer Chemother 2006, 33, 337–343. [Google Scholar]
- Lee, S.-W.; Yun, M.-H.; Jeong, S.W.; In, C.-H.; Kim, J.-Y.; Seo, M.-H.; Pai, C.-M.; Kim, S.-O. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J. Control. Release 2011, 155, 262–271. [Google Scholar] [CrossRef]
- Hwang, H.-Y.; Kim, I.-S.; Kwon, I.C.; Kim, Y.-H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2008, 128, 23–31. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Li, R.; Ding, Y.; Qian, X.; Yu, L.; Jiang, X. The antitumor effect of novel docetaxel-loaded thermosensitive micelles. Eur. J. Pharm. Biopharm. 2008, 69, 527–534. [Google Scholar] [CrossRef]
- Liang, G.; Jia-Bi, Z.; Fei, X.; Bin, N. Preparation, characterization and pharmacokinetics of N-palmitoyl chitosan anchored docetaxel liposomes. J. Pharm. Pharmacol. 2007, 59, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.; Leroux, J.-C.; Allen, C. Enhancement of docetaxel solubility via conjugation of formulation-compatible moieties. Org. Biomol. Chem. 2009, 7, 3437–3446. [Google Scholar] [CrossRef]
- Shaikh, M.; Choudhury, N.R.; Knott, R.; Garg, S. Engineering stent based delivery system for esophageal cancer using docetaxel. Mol. Pharm. 2015, 12, 2305–2317. [Google Scholar] [CrossRef]
- Ilson, D.H. Esophageal cancer chemotherapy: Recent advances. Gastrointest. Cancer Res. GCR 2008, 2, 85–92. [Google Scholar]
- Sánchez-Moreno, P.; Boulaiz, H.; Ortega-Vinuesa, J.L.; Peula-García, J.M.; Aránega, A. Novel drug delivery system based on docetaxel-loaded nanocapsules as a therapeutic strategy against breast cancer cells. Int. J. Mol. Sci. 2012, 13, 4906–4919. [Google Scholar] [CrossRef]
- Mohsin, S.; Arellano, I.H.; Choudhury, N.R.; Garg, S. Docetaxel epimerization in silicone films: A case of drug excipient incompatibility. Drug Test. Anal. 2014, 6, 1076–1084. [Google Scholar] [CrossRef]
- Shepherd, F.; Dancey, J.; Ramlau, R.; Mattson, K.; Gralla, R.; O’Rourke, M.; Levitan, N.; Gressot, L.; Vincent, M.; Burkes, R.; et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 2095–2103. [Google Scholar] [CrossRef]
- Guo, D.; Han, Y.; Ding, Y.; Fang, S.; Tan, W. Prestrain-free electrostrictive film sandwiched by asymmetric electrodes for out-of-plane actuation. Chem. Eng. J. 2018, 352, 876–885. [Google Scholar] [CrossRef]
- Tang, M.; Hou, J.; Lei, L.; Liu, X.; Guo, S.; Wang, Z.; Chen, K. Preparation, characterization and properties of partially hydrolyzed ethylene vinyl acetate copolymer films for controlled drug release. Int. J. Pharm. 2010, 400, 66–73. [Google Scholar] [CrossRef]
- Sadeghi, M.; Khanbabaei, G.; Dehaghani, A.H.S.; Sadeghi, M.; Aravand, M.A.; Akbarzade, M.; Khatti, S. Gas permeation properties of ethylene vinyl acetate–silica nanocomposite membranes. J. Membr. Sci. 2008, 322, 423–428. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Ferreira, P.; Carvalho, Á.; Correia, T.R.; Antunes, B.P.; Correia, I.J.; Alves, P. Functionalization of polydimethylsiloxane membranes to be used in the production of voice prostheses. Sci. Technol. Adv. Mater. 2013, 14, 055006. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, L.; Hu, M.; Jin, Y.; Han, J. Preparation, Characterization and In Vitro Evaluation of Solid Dispersions Containing Docetaxel. Drug Dev. Ind. Pharm. 2008, 34, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, G.; Vannozzi, L. Fabrication, characterization, and properties of poly (ethylene-co-vinyl acetate) composite thin films doped with piezoelectric nanofillers. Nanomaterials (Basel) 2019, 9, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahiaoui, M.; Denape, J.; Paris, J.Y.; Ural, A.G.; Alcalá, N.; Martínez, F.J. Wear dynamics of a TPU/steel contact under reciprocal sliding. Wear 2014, 315, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Ding, P.; Zhang, M.; Qu, B. Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym. Degrad. Stab. 2007, 92, 1715–1720. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Espinosa, J.I.M.; Cauich-Rodríguez, J.V.; Ávila-Ortega, A.; Vázquez-Torres, H.; Marcos-Fernández, A.; San Román, J. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stab. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Madhavan, K.; Reddy, B.S.R. Synthesis and characterization of poly(dimethylsiloxane-urethane) elastomers: Effect of hard segments of polyurethane on morphological and mechanical properties. J. Polym. Sci. 2006, 44, 2980–2989. [Google Scholar] [CrossRef]
- Zhu, Q.; Feng, S.; Zhang, C. Synthesis and thermal properties of polyurethane–polysiloxane crosslinked polymer networks. J. Appl. Polym. Sci. 2003, 90, 310–315. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar]
- Tallury, P.; Alimohammadi, N.; Kalachandra, S. Poly(ethylene-co-vinyl acetate) copolymer matrix for delivery of chlorhexidine and acyclovir drugs for use in the oral environment: Effect of drug combination, copolymer composition and coating on the drug release rate. Dent. Mate. 2007, 23, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Tomar, R.S.; Deolia, S.K.; Mitra, M.; Mukherjee, R.; Burman, A.C. Isolation and characterization of degradation impurities in docetaxel drug substance and its formulation. J. Pharm. Biomed. Anal. 2007, 43, 1228–1235. [Google Scholar] [CrossRef] [PubMed]







| Polymer Film | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Toughness (MJ m−3) | Young’s Modulus (kPa) |
|---|---|---|---|---|
| PEVA | 3.63 ± 0.21 | 763 ± 21.9 | 1374 ± 11.6 | 10.3 ± 0.57 |
| PEVA1 | 3.14 ± 0.27 | 732 ± 33.6 | 1263 ± 6.35 | 10.7 ± 1.52 |
| PEVA5 | 2.64 ± 0.30 | 730 ± 30.0 | 1062 ± 6.35 | 10.7 ± 0.57 |
| PEVA10 | 2.78 ± 0.16 | 642 ± 16.8 | 983 ± 11.0 | 11.7 ± 1.52 |
| PU | 11.3 ± 0.08 | 247 ± 22.4 | 1385 ± 13.5 | 199 ± 3.60 |
| PU1 | 10.1 ± 0.14 | 237 ± 16.2 | 1479 ± 9.50 | 250 ± 5.00 |
| PU5 | 8.49 ± 0.08 | 241 ± 29.8 | 968 ± 6.50 | 50.0 ± 3.46 |
| PU10 | 8.47 ± 0.37 | 247 ± 21.0 | 892 ± 16.6 | 50.3 ± 5.50 |
| PSi | 2.50 ± 0.82 | 234 ± 22.3 | 214 ± 7.02 | 14.46 ± 2.63 |
| PSi1 | 1.64 ± 0.12 | 169 ± 34.0 | 125 ± 3.51 | 23.60 ± 2.91 |
| PSi5 | 1.62 ± 0.15 | 105 ± 7.96 | 65 ± 5.00 | 13.50 ± 0.27 |
| PSi10 | 1.33 ± 0.34 | 88.9 ± 13.3 | 43 ± 6.02 | 12.38 ± 1.31 |
| DTX-Loaded Polymer Film | Weight of the Film a ± SD (mg) | Film Thickness ± SD (µm) | Theoretical Loading ± SD (µg/cm−2) | Experimental Loading ± SD (µg/cm−2) | Percentage Recovery |
|---|---|---|---|---|---|
| PEVA1 | 30.02 ± 2.00 | 303.33 ± 2.89 | 475.29 ± 6.23 | 448.67 ± 9.97 | 94.4 ± 2.65 |
| PEVA5 | 30.23 ± 0.92 | 306.67 ± 7.64 | 2476.5 ± 4.26 | 2387.4 ± 3.83 | 96.4 ± 0.32 |
| PEVA10 | 31.77 ± 1.78 | 302.44 ± 5.08 | 5228.2 ± 8.30 | 4942.0 ± 12.28 | 94.5 ± 0.19 |
| PU1 | 28.50 ± 1.58 | 296.67 ± 5.77 | 447.05 ± 0.95 | 411.92 ± 0.50 | 92.1 ± 0.93 |
| PU5 | 29.78 ± 1.85 | 311.67 ± 10.41 | 2383.5 ± 4.72 | 2273.0 ± 4.13 | 95.4 ± 0.13 |
| PU10 | 30.09 ± 2.48 | 302.45 ± 8.45 | 5031.8 ± 3.17 | 4614.1 ± 3.77 | 91.7 ± 0.21 |
| PSi1 | 35.57 ± 3.26 | 316.67 ± 15.28 | 325.50 2.36 | 312.46 ± 6.36 | 96.0 ± 1.60 |
| PSi5 | 36.67 ± 2.42 | 325.00 ± 13.23 | 2041.5 ± 1.91 | 1915.8 ± 11.58 | 93.8 ± 0.50 |
| PSi10 | 38.04 ± 4.28 | 300.78 ± 12.54 | 3564.0 ± 3.99 | 3245.6 ± 10.16 | 91.1 ± 0.20 |
| Sample | Higuchi | First-Order | Zero-Order | Hixon–Crowell | Kors–Peppas |
|---|---|---|---|---|---|
| (R2) | (R2) | (R2) | (R2) | (R2) | |
| PEVA5 | 0.6271 | 0.5417 | 0.4069 | 0.493 | 0.966 |
| PU5 | 0.9950 | 0.9613 | 0.9151 | 0.948 | 0.982 |
| PSi5 | 0.9922 | 0.9672 | 0.9621 | 0.965 | 0.987 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouladian, P.; Afinjuomo, F.; Arafat, M.; Bergamin, A.; Song, Y.; Blencowe, A.; Garg, S. Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents. Pharmaceutics 2020, 12, 444. https://doi.org/10.3390/pharmaceutics12050444
Fouladian P, Afinjuomo F, Arafat M, Bergamin A, Song Y, Blencowe A, Garg S. Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents. Pharmaceutics. 2020; 12(5):444. https://doi.org/10.3390/pharmaceutics12050444
Chicago/Turabian StyleFouladian, Paris, Franklin Afinjuomo, Mohammad Arafat, Amanda Bergamin, Yunmei Song, Anton Blencowe, and Sanjay Garg. 2020. "Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents" Pharmaceutics 12, no. 5: 444. https://doi.org/10.3390/pharmaceutics12050444
APA StyleFouladian, P., Afinjuomo, F., Arafat, M., Bergamin, A., Song, Y., Blencowe, A., & Garg, S. (2020). Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents. Pharmaceutics, 12(5), 444. https://doi.org/10.3390/pharmaceutics12050444