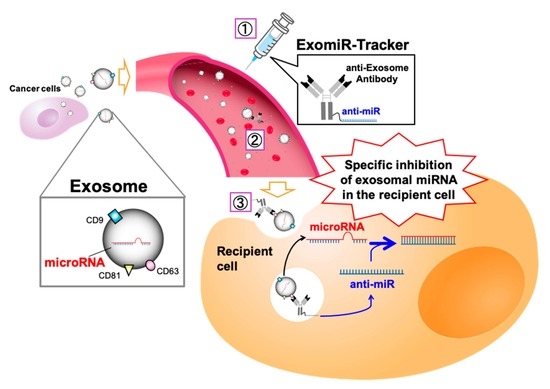
Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ExomiR-Tracker
2.2. Cell Lines
2.3. Confocal Microscopy
2.4. Luciferase Reporter Assay
2.5. Exosome Isolation and qRT-PCR
2.6. Scratch Assay
2.7. In Vivo Study
3. Results and Discussion
3.1. Cellular Uptake of Anti-Exosome Antibodies
3.2. Cellular Uptake and Localization of ExomiR-Trackers
3.3. Evaluation of Inhibitory Effects of ExomiR-Trackers on MiR-21 Functions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, M.; Okada, F.; Ochiya, T. miRNA therapy targeting cancer stem cells: A new paradigm for cancer treatment and prevention of tumor recurrence. Ther. Deliv. 2015, 6, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Zhang, X.; Gu, H.; Ma, J.; Wen, X.; Zhou, J.; Qian, H.; Xu, W.; Qian, J.; Lin, J. miR-374a-5p: A new target for diagnosis and drug resistance therapy in gastric cancer. Mol. Ther. Nucleic Acids 2019, 18, 320–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; She, J.; Chen, X.; Zhang, Q.; Zhang, X.; Wang, Y.; Ye, J.; Shi, J.; Tao, J.; Feng, M.; et al. Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine 2019, 19, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niel, G.V.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Ye, M.; Wu, J.; Ma, L.; Chen, H. Cisplatin-resistant MDA-MB-231 cell-derived exosomes increase the resistance of recipient cells in an exosomal miR-423-5p-dependent manner. Curr. Drug Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Kuwajima, S.; Braich, R.; Rajeev, K.G.; Pena, J.; Tuschl, T.; Manoharan, M.; Stoffel, M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007, 35, 2885–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miroshnichenko, S.K.; Patutina, O.A.; Burakova, E.A.; Chelobanov, B.P.; Fokina, A.A.; Vlassov, V.V.; Altman, S.; Zenkova, M.A.; Stetsenko, D.A. Mesyl phosphoramidate antisense oligonucleotides as an alternative to phosphorothioates with improved biochemical and biological properties. Proc. Natl. Acad. Sci. USA 2019, 116, 1229–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, Y.; Takata, K.; Nakagawa, Y.; Kawakami, H.; Fujioka, S.; Kobayashi, K.; Hattori, Y.; Kitamura, Y.; Akaji, K.; Ashihara, E. Effective internalization of U251-MG-secreted exosomes into cancer cells and characterization of their lipid components. Biochem. Biophys. Res. Commun. 2015, 456, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic, M.M.; Molina, H.; Kohsaka, S.; Giannatale, A.D.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [PubMed] [Green Version]
- Ma, Y.; Kowolik, C.M.; Swiderski, P.M.; Kortylewski, M.; Yu, H.; Horne, D.A.; Jove, R.; Caballero, O.L.; Simpson, A.J.; Lee, F.T.; et al. Humanized Lewis-Y specific antibody based delivery of STAT3 siRNA. ACS Chem. Biol. 2011, 6, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyoshi, J.; Matsuyama, Y.; Kobori, A.; Murakami, A.; Sugiyama, H.; Yamayoshi, A. Effective Anti-miRNA oligonucleotides show high releasing rate of microRNA from RNA-induced silencing complex. Nucleic Acid Ther. 2017, 27, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Gupta, D.; Shankar, S.; Srivastava, R.K. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget 2015, 6, 3280–3291. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamayoshi, A.; Oyama, S.; Kishimoto, Y.; Konishi, R.; Yamamoto, T.; Kobori, A.; Harada, H.; Ashihara, E.; Sugiyama, H.; Murakami, A. Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA. Pharmaceutics 2020, 12, 545. https://doi.org/10.3390/pharmaceutics12060545
Yamayoshi A, Oyama S, Kishimoto Y, Konishi R, Yamamoto T, Kobori A, Harada H, Ashihara E, Sugiyama H, Murakami A. Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA. Pharmaceutics. 2020; 12(6):545. https://doi.org/10.3390/pharmaceutics12060545
Chicago/Turabian StyleYamayoshi, Asako, Shota Oyama, Yusuke Kishimoto, Ryo Konishi, Tsuyoshi Yamamoto, Akio Kobori, Hiroshi Harada, Eishi Ashihara, Hiroshi Sugiyama, and Akira Murakami. 2020. "Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA" Pharmaceutics 12, no. 6: 545. https://doi.org/10.3390/pharmaceutics12060545
APA StyleYamayoshi, A., Oyama, S., Kishimoto, Y., Konishi, R., Yamamoto, T., Kobori, A., Harada, H., Ashihara, E., Sugiyama, H., & Murakami, A. (2020). Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA. Pharmaceutics, 12(6), 545. https://doi.org/10.3390/pharmaceutics12060545