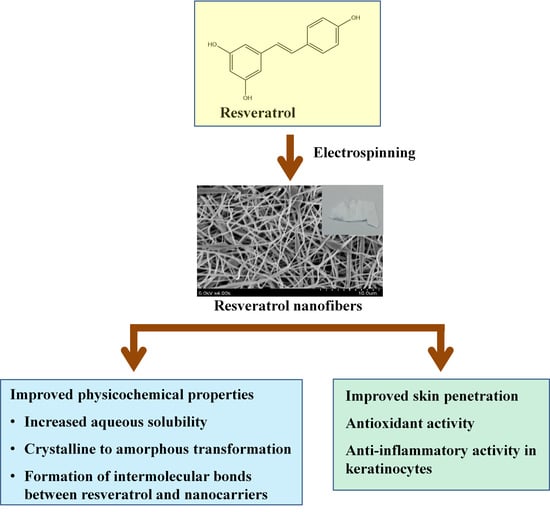
Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Procedure
2.3. High-Performance Liquid Chromatography
2.4. Determination of Entrapment Efficiency
2.5. Determination of Aqueous Solubility
2.6. Scanning Electron Microscopy
2.7. X-Ray Diffractometry
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.9. DPPH Free Radical Scavenging Activity
2.10. Reducing Power Assay
2.11. ABTS Cation Free Radical Scavenging Activity
2.12. Ex Vivo Skin Penetration
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
3.1. Entrapment Efficiency and Aqueous Solubility
3.2. Surface Morphology of Resveratrol and Resveratrol Nanofibers
3.3. Crystalline versus Amorphous Structure of Resveratrol and Resveratrol Nanofibers
3.4. Intermolecular Chemical Bond Formation Between Resveratrol, HPBCD and PVP
3.5. DPPH Free Radical Scavenging Activity
3.6. Reducing Power
3.7. ABTS Cation Free Radical Scavenging Activity
3.8. Ex Vivo Skin Penetration
3.9. Effects of Resveratrol Nanofibers on Particulate Matter (PM)-Induced Inflammatory Proteins Expression in HaCaT Keratinocytes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucciolla, V.; Borriello, A.; Oliva, A.; Galletti, P.; Zappia, V.; Della Ragione, F. Resveratrol: From basic science to the clinic. Cell Cycle 2007, 6, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar] [PubMed]
- Csiszar, A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, J.; Shi, J.S. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef]
- Wu, J.M.; Wang, Z.R.; Hsieh, T.C.; Bruder, J.L.; Zou, J.G.; Huang, Y.Z. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Et Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.W.; Aziz, M.H. Protective molecular mechanisms of Resveratrol in UVR induced Skin carcinogenesis. Photodermatol. Photoimmunol. Photomed. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitlock, N.C.; Baek, S.J. The anticancer effects of resveratrol: Modulation of transcription factors. Nutr. Cancer 2012, 64, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, T.C.; Wu, J.M. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors 2010, 36, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [Green Version]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 2006, 11, 217–225. [Google Scholar]
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998, 138, 619–620. [Google Scholar] [CrossRef] [Green Version]
- Bolko, K.; Zvonar, A.; Gasperlin, M. Mixed lipid phase SMEDDS as an innovative approach to enhance resveratrol solubility. Drug Dev. Ind. Pharm. 2014, 40, 102–109. [Google Scholar] [CrossRef]
- Balata, G.F.; Essa, E.A.; Shamardl, H.A.; Zaidan, S.H.; Abourehab, M.A. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des. Dev. Ther. 2016, 10, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.O.; Lee, J.S.; Lee, H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and gamma-poly (glutamic acid). Colloids Surf. B Biointerfaces 2016, 147, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhong, L.X.; Zhan, Z.Y.; Huang, Z.H.; Xiong, J.P. Enhanced antitumor efficacy of resveratrol-loaded nanocapsules in colon cancer cells: Physicochemical and biological characterization. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 375–382. [Google Scholar] [PubMed]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Qu, Z.; Pujara, N.; Sheng, Y.; Jambhrunkar, S.; McGuckin, M.; Popat, A. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surf. B Biointerfaces 2016, 144, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharmscitech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release Off. J. Control. Release Soc. 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Chew, S.Y.; Wen, Y.; Dzenis, Y.; Leong, K.W. The role of electrospinning in the emerging field of nanomedicine. Curr. Pharm. Des. 2006, 12, 4751–4770. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.G.; Wang, M.; Li, X.; Liu, X.; Zhu, L.M.; Annie Bligh, S.W. Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1601. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and In-Vitro characterization of chitosan-nanoparticles loaded with the iron chelator deferoxamine mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cosmetics_Europe. COLIPA Guidelines: Guidelines for Percutaneous Absorption/Penetration. 1997. Available online: https://www.cosmeticseurope.eu/files/8314/6407/9075/Guidelines_for_Percutaneous_Absorption-Penetration_-_1997.pdf (accessed on 2 June 2020).
- Martínez-Ortega, L.; Mira, A.; Fernandez-Carvajal, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Development of a new delivery system based on drug-loadable electrospun nanofibers for psoriasis treatment. Pharmaceutics 2019, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Karavas, E.; Georgarakis, M.; Docoslis, A.; Bikiaris, D. Combining SEM, TEM, and micro-Raman techniques to differentiate between the amorphous molecular level dispersions and nanodispersions of a poorly water-soluble drug within a polymer matrix. Int. J. Pharm. 2007, 340, 76–83. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Wang, W.; Winstead, D.A. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J. Pharm. Sci. 2009, 98, 2422–2431. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015, 487, 213–222. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPbetaCD nanofibers for oral candidiasis application. Pharm. Res. 2014, 31, 1893–1906. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Panomsuk, S.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of mucoadhesive chitosan coated polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for oral candidiasis. Carbohydr. Polym. 2015, 132, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Tonglairoum, P.; Chuchote, T.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Encapsulation of plai oil/2-hydroxypropyl-beta-cyclodextrin inclusion complexes in polyvinylpyrrolidone (PVP) electrospun nanofibers for topical application. Pharm. Dev. Technol. 2014, 19, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. The use of chemically modified cyclodextrins in the development of formulations for chemical delivery systems. Pharmazie 2002, 57, 94–101. [Google Scholar] [PubMed]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic acid-the little-known antioxidant in woody plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [Green Version]
- Trommer, H.; Neubert, R.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharm. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Lee, K.E.; Ryu, J.J.; Jo, Y.K.; Yeo, H.; Kang, S. 2′-Fucosyllactose attenuates particulate matter-induced inflammation via inhibition of hypoxia-inducible factor in keratinocytes. Biol. Pharm. Bull. 2019, 42, 1620–1627. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.M.; Liao, L.Y.; Li, L.; Yi, F.; Meng, H.; He, Y.F.; Guo, M.M. Skin inflammation induced by ambient particulate matter in China. Sci. Total. Env. 2019, 682, 364–373. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Kim, J.M.; Lee, M.K.; Seo, S.J.; Park, K.Y. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int. J. Mol. Med. 2019, 43, 2516–2522. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Derm. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Rajakariar, R.; Yaqoob, M.M.; Gilroy, D.W. COX-2 in inflammation and resolution. Mol. Interv. 2006, 6, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Corbel, M.; Belleguic, C.; Boichot, E.; Lagente, V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol. Toxicol. 2002, 18, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hannocks, M.J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Damme, J.; Opdenakker, G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 2014, 214, 193–206. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS Pharmscitech 2010, 11, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Derm. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]









| Ratio (Res:PVP:HPBCD, W/W/W) | Resveratrol (g) | PVP (g) | HPBCD (g) |
|---|---|---|---|
| 1:4:20 | 0.05 | 0.2 | 1 |
| 1:8:20 | 0.05 | 0.4 | 1 |
| 1:16:20 | 0.05 | 0.8 | 1 |
| 1:4:40 | 0.05 | 0.2 | 2 |
| 1:8:40 | 0.05 | 0.4 | 2 |
| 1:16:40 | 0.05 | 0.8 | 2 |
| Ratio (Res:PVP:HPBCD, W/W/W) | Entrapment Efficiency (%) | Solubility (μg/mL) |
|---|---|---|
| 1:04:20 | 98.27 ± 4.59 | 894.90 ± 22.02 |
| 1:08:20 | 84.54 ± 5.98 | 875.68 ± 23.83 |
| 1:16:20 | 79.00 ± 8.21 | 855.50 ± 35.20 |
| 1:04:40 | 97.31 ± 5.24 | 860.34 ± 22.30 |
| 1:08:40 | 96.01 ± 5.60 | 900.63 ± 31.47 |
| 1:16:40 | 90.16 ± 7.67 | 896.43 ± 25.46 |
| Pure resveratrol | - | 0.041 ± 0.001 |
| Ratio (Res:PVP:HPBCD, W/W/W) | Nanofiber Diameter (nm) |
|---|---|
| 1:04:20 | 437.96 ± 41.92 |
| 1:08:20 | 578.66 ± 40.11 |
| 1:16:20 | 906.51 ± 61.85 |
| 1:04:40 | 835.78 ± 79.57 |
| 1:08:40 | 1259.59 ± 44.29 |
| 1:16:40 | 2639.59 ± 157.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. https://doi.org/10.3390/pharmaceutics12060552
Lin Y-C, Hu SC-S, Huang P-H, Lin T-C, Yen F-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics. 2020; 12(6):552. https://doi.org/10.3390/pharmaceutics12060552
Chicago/Turabian StyleLin, Ying-Cheng, Stephen Chu-Sung Hu, Pao-Hsien Huang, Tzu-Ching Lin, and Feng-Lin Yen. 2020. "Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications" Pharmaceutics 12, no. 6: 552. https://doi.org/10.3390/pharmaceutics12060552
APA StyleLin, Y. -C., Hu, S. C. -S., Huang, P. -H., Lin, T. -C., & Yen, F. -L. (2020). Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics, 12(6), 552. https://doi.org/10.3390/pharmaceutics12060552