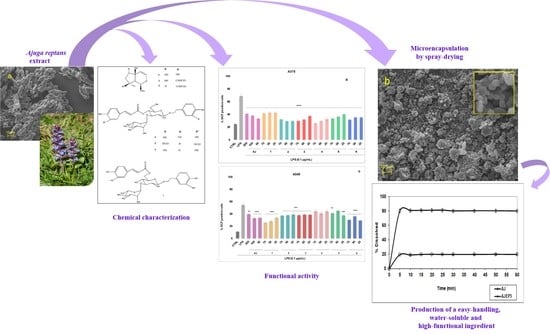
Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. General Experimental Procedures
2.3. Plant Material and Extract Preparation
2.4. Isolation Procedures and Chemical Characterization of AJ Compounds
2.5. Quantitative Determination of Total Phenol Content
2.6. Quantitative HPLC Analysis
2.7. Chemical-Based Free Radical Scavenging Activity
2.7.1. Diphenyl-2-Picrylhydrazyl Radical (DPPH) Test
2.7.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.8. Antioxidant Activity in Cell Systems
2.8.1. Cell Culture
2.8.2. Cell Viability
2.8.3. Cell Treatment
2.8.4. Antioxidant Activity
2.9. Microencapsulation Process
Polymeric Matrix Preparation and Spray Drying Conditions
2.10. Powders Characterization
2.10.1. Yield and Loading Efficacy
2.10.2. Quantitative Analysis
HPLC Method
UV Method
2.10.3. Morphology and Particle size Distribution
2.10.4. Dissolution/Release Test
2.10.5. Differential Scanning Calorimetry (DSC)
2.11. Study of AJ Chemical Stability and AJEP3 Functional Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Ajuga reptans L. Extract Preparation, Chemical Composition, and Quantitative Analysis
3.2. Chemical-Based Free Radical Scavenging Activity
3.3. Antioxidant Activity in Cell Systems
3.4. Microencapsulation Process
3.4.1. Process Efficiency
3.4.2. Powder Characterization
Solid-State, Morphology, and Particle size
Thermal Analyses
Physical Stability
3.4.3. In Vitro Dissolution/Release Tests
3.5. Chemical Stability and Evaluation of the Functional Activity of AJEP3
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zafar, M.S.; Quarta, A.; Marradi, M.; Ragusa, A. Recent developments in the reduction of oxidative stress through antioxidant polymeric formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanari, E.; Di Meo, C.; Coviello, T.; Gueguen, V.; Pavon-Djavid, G.; Matricardi, P. Intracellular delivery of natural antioxidants via hyaluronan nanohydrogels. Pharmaceutics 2019, 11, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, F.; Esposito, T.; Mencherini, T.; Piccinelli, A.L.; Gazzerro, P.; Picerno, P.; Russo, P.; Del Gaudio, P.; Essolito, M.; Campiglia, P.; et al. Annurca peel extract: From the chemical composition, through the functional activity, to the formulation and characterisation of a topical oil-in-water emulsion. Nat. Prod. Res. 2016, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm. Sci. 2009, 22, 425–462. [Google Scholar]
- Luan, F.; Han, K.; Li, M.; Zhang, T.; Liu, D.; Yu, L.; Lv, H. Ethnomedicinal uses, phytochemistry, pharmacology, and toxicology of species from the genus Ajuga L.: A systematic review. Am. J. Chin. Med. 2019, 47, 959–1003. [Google Scholar] [CrossRef]
- Ni, B.; Dong, X.; Fu, J.; Yin, X.; Lin, L.; Xia, Z.; Zhao, Y.; Xue, D.; Yang, C.; Ni, J. phytochemical and biological properties of ajuga decumbens (Labiatae): A review. Trop. J. Pharm. Res. 2015, 14, 1525–1536. [Google Scholar] [CrossRef] [Green Version]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef]
- Giovanni Aliotta, A.P. Useful Plants in Renal Therapy According to Plinythe Elder. Am. J. Nephrol. 1994, 14, 399–411. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.M.; Moldovan, C.; Oniga, I. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and in Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. And A. Reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Furusawa, C.; Ozono, T.; Oda, K.; Yasuda, S.; Okawa, M.; Kinjo, J.; Ikeda, T.; Miyashita, H.; Yoshimitsu, H.; et al. Four new iridoid glucosides from Ajuga reptans. Chem. Pharm. Bull. 2011, 59, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Toiu, A.; Vlase, L.; Gheldiu, A.M.; Vodnar, D.; Oniga, I. Evaluation of the antioxidant and antibacterial potential of bioactive compounds from Ajuga reptans extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Vertuani, S.; Ziosi, P.; Toso, R.D.; Vicentini, C.B.; Manfredini, S. Dualistic Properties of Cosmetic Formulations Based on Phenylpropanoids from Ajuga reptans. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, F.; Picerno, P.; Mencherini, T.; Russo, P.; Gasparri, F.; Giannini, V.; Lauro, M.R.; Puglisi, G.; Aquino, R.P. Enhanced technological and permeation properties of a microencapsulated soy isoflavones extract. J. Food Eng. 2013. [Google Scholar] [CrossRef]
- Picerno, P.; Sansone, F.; Mencherini, T.; Prota, L.; Aquino, R.P.; Rastrelli, L.; Lauro, M.R. Citrus bergamia juice: Phytochemical and technological studies. Nat. Prod. Commun. 2011, 6, 951–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients—A Review. Food Bioprocess. Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Esposito, T.; Mencherini, T.; Del Gaudio, P.; Auriemma, G.; Franceschelli, S.; Picerno, P.; Aquino, R.P.; Sansone, F. Design and development of spray-dried microsystems to improve technological and functional properties of bioactive compounds from hazelnut shells. Molecules 2020, 25, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Davoodipoor, A.M.; Sadati, S.N.; Ardekani, M.R.S.; Sharifzadeh, M. Antinociceptive effect of some extracts from Ajuga chamaecistus Ging. ssp. tomentella (Boiss.) Rech. f. aerial parts. DARU J. Pharm. Sci. 2014, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lamardi, S.N.S.; Tahroodi, S.; Khanavi, M.; Doust, R.H. Antibacterial Activity of Aerial Part Extracts and Fractions of Ajuga chamaecistus ssp. tomentella. Tradit. Integr. Med. 2017, 2, 61–66. [Google Scholar]
- Chaudhuri, R.K.; Afifi-Yazar, F.Ü.; Sticher, O.; Winkler, T. 13C NMR spectroscopy of naturally occurring iridoid glucosides and their acylated derivatives. Tetrahedron 1980, 36, 2317–2326. [Google Scholar] [CrossRef]
- Oganesyan, G.B.; Galstyan, A.M.; Mnatsakanyan, V.A.; Shashkov, A.S.; Agababyan, P. Phenylpropanoid glycosides of Teucrium polium. Chem. Nat. Compd. 1991, 27, 556–559. [Google Scholar] [CrossRef]
- Mansoor Ahmad, O.S. Isolation of acacetin-7-O-rutinoside and martynoside from Buddleja davidii. J. Chem. Soc. Pak. 1988, 10, 117–123. [Google Scholar]
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Moccia, S.; Volpe, M.G.; Sansone, F.; Di Stasio, M.; Salvatore, P.; Russo, G.L.; Aquino, R.P.; Pagliarulo, C. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess. Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-rich Fraction with Antioxidant and Anti-Candida albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa miller.) burs extracts and functional compounds: Uhplc-uv-hrms profiling, antioxidant activity, and inhibitory effects on phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerbab, K.; Sansone, F.; Zaiter, L.; Esposito, T.; Celano, R.; Franceschelli, S.; Pecoraro, M.; Benayache, F.; Rastrelli, L.; Picerno, P.; et al. Halimium halimifolium: From the Chemical and Functional Characterization to a Nutraceutical Ingredient Design 1. Planta Med. 2019, 85, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Esposito, T.; Mencherini, T.; Lauro, M.R.; Del Gaudio, P.; Picerno, P.; Pepe, G.; Aquino, R.P. Particle technology applied to a lactose/NaCMC blend: Production and characterization of a novel and stable spray-dried ingredient. Powder Technol. 2018, 329, 304–312. [Google Scholar] [CrossRef]
- Farmacopea Ufficiale Italiana XII edizione Istituto poligrafico dello stato Roma 2008 Farmacopea Ufficiale Italiana. Available online: https://www.file-pdf.it/2015/04/27/133201093-farmacopea-12/133201093-farmacopea-12.pdf (accessed on 14 July 2020).
- Agency European Medicines ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products; Agency European Medicines: London, UK, 2006.
- Budzianowski, J.; Skrzypczak, L. Phenylpropanoid esters from Lamium album flowers. Phytochemistry 1995, 38, 997–1001. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Ariburnu, E.; Masullo, M.; Festa, M.; Capasso, A.; Yesilada, E.; Piacente, S. Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 2012, 83, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Minghetti, A.; Crespi Perellino, N.; Roda, A.; Danieli, B.; Frigerio, G. Compounds with an Antioxidant Activity, Compositions Useful as Food Integrators Containing Them and Process for Their Preparation. U.S. Patent 6,544,965, 8 April 2003. [Google Scholar]
- Dal Monte, R.; Dal Toso, R.; Minghetti, A.; Crespi Perellino, N.; Pressi, G. Extracts from Ajuga Reptans Cell Lines, Their Preparation and Use. U.S. Patent 9,623,063, 18 April.
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef]
- Lee, J.K.M.; Taip, F.S.; Abdulla, H.Z. Effectiveness of additives in spray drying performance: A review. Food Res. 2018, 2, 486–499. [Google Scholar] [CrossRef]
- Sansone, F.; Esposito, T.; Lauro, M.R.; Picerno, P.; Mencherini, T.; Gasparri, F.; De Santis, S.; Chieppa, M.; Cirillo, C.; Aquino, R.P. Application of spray drying particle engineering to a high-functionality/low-solubility milk thistle extract: Powders production and characterization. Molecules 2018, 23, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, V.; Bhandari, B.R.; Howes, T. Optimization of co-current spray drying process of sugar-rich foods. Part I-Moisture and glass transition temperature profile during drying. J. Food Eng. 2005, 71, 55–65. [Google Scholar] [CrossRef]
- Neacsu, A.; Gheorghe, D.; Marinescu, C.; Stancu, E.; Tecuceanu, V.; Ciuculescu, C. The effect of gamma rays upon L-proline and 4-hydroxy-L-proline. A thermochemical study. Radiat. Phys. Chem. 2019, 156, 115–127. [Google Scholar] [CrossRef]
- Einfal, T.; Planinšek, O.; Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Gavarić, A.; Vladić, J.; Ambrus, R.; Jokić, S.; Szabó-Révész, P.; Tomić, M.; Blažić, M.; Vidović, S. Spray drying of a subcritical extract using marrubium vulgare as a method of choice for obtaining high quality powder. Pharmaceutics 2019, 11, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutz, J.K.; Zambiazi, R.C.; Borges, C.D.; Krumreich, F.D.; Da Luz, S.R.; Hartwig, N.; Da Rosa, C.G. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr. Polym. 2013, 98, 1256–1265. [Google Scholar] [CrossRef]
- Pereira Souza, A.C.; Deyse Gurak, P.; Damasceno Ferreira Marczak, L. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Human, C.; de Beer, D.; Aucamp, M.; Marx, I.J.; Malherbe, C.J.; Viljoen-Bloom, M.; van der Rijst, M.; Joubert, E. Preparation of rooibos extract-chitosan microparticles: Physicochemical characterisation and stability of aspalathin during accelerated storage. LWT 2020, 117, 108653. [Google Scholar] [CrossRef]














| Sample | P/HEC g/100 mL | Water/EtOH Solvent System | L g/100 mL | AJ g/100 mL | Yield % | TEC a % | TAC b % | AEC c % | AAC d % | EE e % | d50 µm (span) f,e |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AJ raw | - | - | - | - | - | - | - | 2.80 ± 0.5 d | - | 165.1 (2.02) | |
| HEC | - | - | - | - | - | - | - | - | - | 277.22 (1.60) | |
| P | - | - | - | - | - | - | - | - | - | 250.10 (1.71) | |
| EP1 | 5.0/0.2 | 100/0 | - | 32.00 ± 1.14 | - | - | - | - | - | - | |
| EP2 | 5.0/0.2 | 100/0 | 0.2 | 51.28 ± 1.36 | - | - | - | - | |||
| EP3 | 5.0/0.2 | 80/20 | 0.2 | - | 85.70 ± 1.91 | - | - | - | - | - | 102.93 (1.44) |
| EP4 | 5.0/1.25 | H2O | - | - | 10.31 ± 1.14 | ||||||
| EP5 | 5.0/0.5 | H2O | - | - | 20.66 ± 1.22 | ||||||
| AJEP1 | 5.0/0.2 | 100/0 | - | 0.55 | 32.76 ± 1.91 | 9.56 ± 0.31 f | 0.27 ± 0.05 f | 5.41 ± 0.63 | 0.14 ± 0.08 | 56.6 | n.d. |
| AJEP2 | 5.0/0.2 | 100/0 | 0.2 | 0.50 | 41.00 ± 2.42 | 8.50 ± 0.64 f | 0.25 ± 0.04 f | 6.33 ± 0.71 | 0.18 ± 0.12 | 71.6 | n.d. |
| AJEP3 | 5.0/0.2 | 80/20 | 0.2 | 0.58 | 71.50 ± 1.42 | 9.70 ± 0.37 f | 0.27 ± 0.02 | 9.69 ± 0.32 | 0.27 ± 0.06 | 99.9 | 18.46 (1.63) |
| t0 | t6months | t0 | t6months | t0 | t6months | |
|---|---|---|---|---|---|---|
| Samples | AAC% a | DPPH Test EC50 b,c | TEAC Test d,e | |||
| AJ unprocessed extract | 2.80 ± 0.40 | 1.23 ± 0.15 * | 135.78 ± 4.12 | 161.71 ± 2.11 * | 0.29 ± 0.01 | 0.14 ± 0.06 * |
| AJEP3 | 2.70 ± 0.06 | 2.50 ± 0.09 | 130.11 ± 0.04 | 127.66 ± 0.02 | 0.22 ± 0.03 | 0.21 ± 0.02 |
| Teupolioside f | 9.18 ± 1.32 | 7.12 ± 0.80 | 0.64 ± 0.04 | 0.58 ± 0.04 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, T.; Sansone, F.; Auriemma, G.; Franceschelli, S.; Pecoraro, M.; Picerno, P.; Aquino, R.P.; Mencherini, T. Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes. Pharmaceutics 2020, 12, 671. https://doi.org/10.3390/pharmaceutics12070671
Esposito T, Sansone F, Auriemma G, Franceschelli S, Pecoraro M, Picerno P, Aquino RP, Mencherini T. Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes. Pharmaceutics. 2020; 12(7):671. https://doi.org/10.3390/pharmaceutics12070671
Chicago/Turabian StyleEsposito, Tiziana, Francesca Sansone, Giulia Auriemma, Silvia Franceschelli, Michela Pecoraro, Patrizia Picerno, Rita P. Aquino, and Teresa Mencherini. 2020. "Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes" Pharmaceutics 12, no. 7: 671. https://doi.org/10.3390/pharmaceutics12070671
APA StyleEsposito, T., Sansone, F., Auriemma, G., Franceschelli, S., Pecoraro, M., Picerno, P., Aquino, R. P., & Mencherini, T. (2020). Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes. Pharmaceutics, 12(7), 671. https://doi.org/10.3390/pharmaceutics12070671