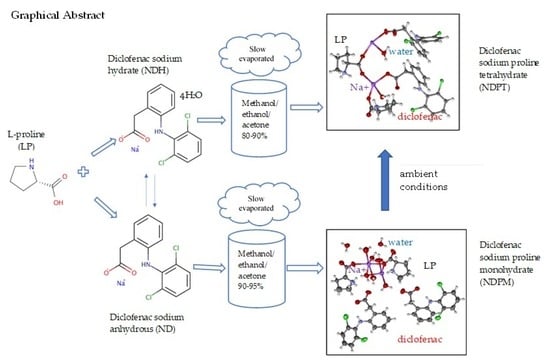
Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of Starting Materials
2.2.2. Screening to Determine the Optimal Molar Ratio of Cocrystals Using a Binary Phase Diagram
2.2.3. Preparation of NDP Cocrystals
2.2.4. Electrothermal Measurement
2.2.5. Crystal Habit Observation
2.2.6. FTIR Measurement
2.2.7. DSC Measurement
2.2.8. DTA/TG Measurement
2.2.9. PXRD Measurement
2.2.10. Crystal Structure Analysis Using Single-Crystal X-ray Diffraction Analysis (SCXRD)
2.2.11. Stability Test
2.2.12. Solubility Test
2.2.13. Dissolution Test
3. Results and Discussion
3.1. ND and NDH Preparation
3.2. Determination of Molar Ratio in NDP for Cocrystallization
3.3. NDP Cocrystals Preparation and Characterization
3.3.1. Crystal Habit Observation
3.3.2. FTIR Measurement Data
3.3.3. Thermal Analysis
3.3.4. PXRD Data
3.4. NDPT Crystal Structure Determination
3.5. Diclofenac Sodium-Proline Monohydrate Salt Cocrystal Isolation, Characterization, and Structure Determination
3.6. Stability Testing
3.6.1. Thermal Profile Analysis on Dried Stability Test Samples
3.6.2. Thermal Profile Analysis on Humidified Stability Test Samples
3.6.3. PXRD Analysis of Stability Test Samples after Drying and Humidifying
3.7. Pharmaceutics Performance Tests
3.7.1. Solubility Testing
3.7.2. Dissolution Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ND | natrium diclofenac (=diclofenac sodium) |
| NDH | natrium diclofenac hydrate (=diclofenac sodium hydrate) |
| LP | L-proline |
| NDP | natrium diclofenac proline |
| NDPT | natrium diclofenac proline tetrahydrate |
| NDPM | natrium diclofenac proline monohydrate |
| DSC | differential scanning calorimetry |
| DTA | differential thermal analysis |
| TG | thermogravimetry |
| FTIR | Fourier transform infrared |
| PXRD | powder X-ray diffractometer/powder X-ray diffractometry |
| SCXRD | single crystal X-ray diffractometry/single crystal X-ray diffractometer |
| RH | relative humidity |
References
- Arisoy, G.G.; Dural, E.; Mergen, G.; Arisoy, M.; Guvendik, G.; Soylemezoglu, T. Development and validation HPLC-UV method for the determination of diclofenac in human plasma with application to pharmacokinetic study. Turk. J. Pharm. Sci. 2016, 13, 292–299. [Google Scholar]
- McGettigan, P.; Henry, D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: An examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med. 2013, 10, e1001388. [Google Scholar] [CrossRef] [PubMed]
- Vemula, V.R.; Lagishetty, V.; Lingala, S. Solubility enhancement techniques. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 41–51. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, F.L.F.; Carneiro, R.L. Evaluation of analytical tools and multivariate methods for quantification of co-former crystals in ibuprofen-nicotinamide co-crystals. J. Pharm. Biomed. Anal. 2014, 89, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tilborg, A.; Springuel, G.; Norberg, B.; Wouters, J.; Leyssens, T. On the influence of using a zwitterionic coformer for cocrystallization: Structural focus on naproxen–proline cocrystals. CrystEngComm 2013, 15, 3341. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Fukte, S.; Wagh, M.P.; Rawat, S. Coformer selection: An important tool in cocrystal formation. Int. J. Pharm. Pharm. Sci. 2014, 6, 9–14. [Google Scholar]
- Kuminek, G.; Cao, F.; Bahia de Oliveira da Rocha, A.; Gonçalves Cardoso, S.; Rodríguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patole, T.; Deshpande, A. Co-crystallization-a technique for solubility enhacement. Int. J. Pharm. Sci. Res. 2014, 5, 3566–3576. [Google Scholar]
- Barikah, K.Z. Traditional and novel methods for cocrystal formation: A mini review. Syst. Rev. Pharm. 2018, 9, 79–82. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Nat. Bur. Stand-A Phys. Chem. 1977, 81A, 89–96. [Google Scholar] [CrossRef]
- Grifasi, F.; Chierotti, M.R.; Gaglioti, K.; Gobetto, R.; Maini, L.; Braga, D.; Dichiarante, E.; Curzi, M. Using salt cocrystals to improve the solubility of niclosamide. Cryst. Growth Des. 2015, 15, 1939–1948. [Google Scholar] [CrossRef]
- Gunnam, A.; Suresh, K.; Nangia, A. Salts and salt cocrystals of the antibacterial drug pefloxacin. Cryst. Growth Des. 2018, 18, 2824–2835. [Google Scholar] [CrossRef]
- Nugrahani, I.; Pertiwi, E.A.; Putra, O.D. Theophylline-Na-saccharin single crystal isolation for its structure determination. Int. J. Pharm. Sci. 2015, 7, 15–24. [Google Scholar]
- Nugrahani, I.; Utami, D.; Ibrahim, S.; Nugraha, Y.P.; Uekusa, H. Zwitterionic cocrystal of diclofenac and L-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study. Eur. J. Pharm. Sci. 2018, 117, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Magri, A.D.; Magri, A.L. Determination of diclofenac salts in pharmaceutical formulations. Fresenius’ J. Anal. Chem. 1998, 362, 577–582. [Google Scholar] [CrossRef]
- Adeyeye, C.M.; Li, P.K. Diclofenac Sodium. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: London, UK, 1990; Volume 19, pp. 123–140. [Google Scholar]
- Fini, A.; Cavallari, C.; Bassini, G.; Ospitali, F.; Morigi, R. Diclofenac salts, part 7: Are the pharmaceutical salts with aliphatic amines stable? J. Pharm. Sci. 2012, 101, 157–3168. [Google Scholar] [CrossRef] [PubMed]
- Reck, G.; Faust, G.; Dietz, G. X-ray crystallographic studies of diclofenac-sodium-structural analysis of diclofenac-sodium tetrahydrate. Pharmazie 1988, 43, 771–774. [Google Scholar]
- Fini, A.; Garuti, M.; Fazio, G.; Alvarez-Fuentes, J.; Holgado, M.A. Diclofenac salts. I. fractal and thermal analysis of sodium and potassium diclofenac salts. J. Pharm. Sci. 2001, 90, 2049–2057. [Google Scholar] [CrossRef]
- Bartolomei, M.; Bertocchi, P.; Antoniella, E.; Rodomonte, A. Physico-chemical characterisation and intrinsic dissolution studies of a new hydrate form of diclofenac sodium: Comparison with anhydrous form. J. Pharm. Biomed. Anal. 2006, 40, 1105–1113. [Google Scholar] [CrossRef]
- Muangsin, N.; Prajaubsook, M.; Chaichit, N.; Siritaedmukul, K.; Hannongbua, S. Crystal structure of a unique sodium distorted linkage in diclofenac sodium pentahydrate. Anal. Sci. 2002, 18, 967–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolomei, M.; Rodomonte, A.; Antoniella, E.; Minelli, G.; Bertocchi, P. Hydrate modifications of the non-steroidal anti-inflammatory drug diclofenac sodium: Solid-state characterization of a trihydrate form. J. Pharm. Biomed. Anal. 2007, 45, 443–449. [Google Scholar] [CrossRef]
- Llinàs, A.; Burley, J.C.; Box, K.J.; Glen, R.C.; Goodman, J.M. Diclofenac solubility: Independent determination of the intrinsic solubility of three crystal forms. J. Med. Chem. 2007, 50, 979–983. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. In Application of Ionic Liquids in Biotechnology; Springer: Cham, Switzerland, 2018. [Google Scholar]
- L-Proline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/L-proline (accessed on 17 July 2019).
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Guru Row, T.N. Comprehending the formation of eutectics and cocrystals in terms of design and their structural interrelationships. Cryst. Growth. Des. 2014, 14, 4187–4198. [Google Scholar] [CrossRef]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of cocrystal formation on binary phase diagrams using thermal analysis. Pharm. Res. 2013, 30, 70–80. [Google Scholar] [CrossRef]
- Nugrahani, I.; Asyarie, S.; Soewandhi, S.N.; Ibrahim, S. The cold contact methods as a simple drug interaction detection system. Res. Lett. Phys. Chem. 2008, 2008, 169247. [Google Scholar] [CrossRef] [Green Version]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical cocrystals: New solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbacz, P.; Wesolowski, M. DSC, FTIR and Raman Spectroscopy coupled with multivariate analysis in a study of co-crystals of pharmaceutical interest. Molecules 2018, 23, 2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szakonyi, G.; Zelkó, R. Water content determination of superdisintegrants by means of ATR-FTIR Spectroscopy. J. Pharm. Biomed. Anal. 2012, 63, 106–111. [Google Scholar] [CrossRef]
- Jegatheesan, A.; Murugan, J.; Neelakantaprasad, B.; Rajarajan, G. FTIR, PXRD, SEM, RGA investigations of ammonium dihydrogen phosphate (ADP) single crystal. Int. J. Comput. Appl. 2012, 53, 15–18. [Google Scholar]
- Ali, H.R.H.; Alhalaweh, A.; Mendes, N.F.C.; Ribeiro-Claro, P.; Velaga, S.P. Solid-state vibrational spectroscopic investigation of cocrystals and salt of indomethacin. Cryst. Eng. Comm. 2012, 14, 6665. [Google Scholar] [CrossRef]
- Nanjwade, V.; Manvi, F.; Shamrez, A.; Nanjwade, B. Characterization of prulifloxacin-salicylic acid complex by IR, DSC and PXRD. J. Pharm. Biomed. Sci. 2011, 5, 1–6. [Google Scholar]
- Brittain, H.G. Vibrational Spectroscopic study of the cocrystal products formed by cinchona alkaloids with 5-nitrobarbituric acid. J. Spectrosc. 2015, 2015, 340460. [Google Scholar] [CrossRef]
- Ryu, I.S.; Liu, X.; Jin, Y.; Sun, J.; Lee, Y.J. Stoichiometrical analysis of competing intermolecular hydrogen bonds using infrared spectroscopy. RSC Adv. 2018, 8, 23481–23488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.C.; Lin, H.L.; Lin, S.Y. Thermal analysis and FTIR spectral curve-fitting investigation of formation mechanism and stability of indomethacin-saccharin cocrystals via solid-state grinding process. J. Pharm. Biomed. Anal. 2012, 66, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Cugovčan, M.; Jablan, J.; Lovrić, J.; Cinčić, D.; Galić, N.; Jug, M. Biopharmaceutical characterization of praziquantel cocrystals and cyclodextrin complexes prepared by grinding. J. Pharm. Biomed. Anal. 2017, 137, 42–53. [Google Scholar] [CrossRef]
- Salas-Zúñiga, R.; Rodríguez-Ruiz, C.; Höpfl, H.; Morales-Rojas, H.; Sánchez-Guadarrama, O.; Rodríguez-Cuamatzi, P.; Herrera-Ruiz, D. Dissolution advantage of nitazoxanide cocrystals in the presence of cellulosic polymers. Pharmaceutics 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugrahani, I.; Utami, D.; Ayuningtyas, L.; Garmana, A.N.; Oktaviary, R. New preparation method using microwave, kinetics, in vitro dissolution-diffusion, and anti-inflammatory study of diclofenac-proline co-crystal. ChemistrySelect 2019, 4, 13396–13403. [Google Scholar] [CrossRef]
- Roy, L.; Lipert, M.P.; Rodríguez-Hornedo, N. Co-Crystal Solubility and Thermodynamic Stability, Pharmaceutical Salts and Co-Crystals; RCS Publishing: Cambridge, UK, 2012; pp. 249–250. [Google Scholar]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Furuishi, T.; Nagase, H.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Solubility improvement of epalrestat by layered structure formation: Via cocrystallization. Cryst. Eng. Comm. 2017, 19, 2614–2622. [Google Scholar] [CrossRef]
- Putra, D.O.; Umeda, D.; Fujita, E.; Haraguchi, T.; Uchida, T.; Yonemochi, E.; Uekusa, H. Solubility improvement of benexate through salt formation using artificial sweetener. Pharmaceutics 2018, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Eyjolfsson, R. Diclofenac sodium: Oxidative degradation in solution and solid state. Drug Dev. Ind. Pharm. 2000, 26, 451–453. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Madusanka, N.; Jones, W. Cocrystal dissociation in the presence of water: A general approach for identifying stable cocrystal forms. J. Pharm. Sci. 2014, 103, 2865–2870. [Google Scholar] [CrossRef]
- Prenner, E.; Chiu, M. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioall. Sci. 2011, 3, 39–60. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Z.; Duan, J.Z. Dissolution Testing of Solid Products. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: New York, NY, USA, 2017; pp. 355–379. [Google Scholar]
- Shohin, I.E.; Grebenkin, D.Y.; Malashenko, E.A.; Stanishevskii, Y.M.; Ramenskaya, G.V. A brief review of the FDA dissolution methods database. Dissolution Technol. 2016, 23, 6–10. [Google Scholar] [CrossRef]
- Chuasuwan, B.; Binjesoh, V.; Polli, J.E.; Zhang, H.; Amidon, G.L.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; et al. Biowaiver Monographs for immediate release solid oral dosage form: Diclofenac sodium and diclofenac potassium. J. Pharm. Sci. 2009, 98, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Utami, D.; Nugraha, Y.P.; Uekusa, H.; Hasianna, R.; Darusman, A.A. Cocrystal construction between the ethyl ester with parent drug of diclofenac: Structural, stability, and anti-inflammatory study. Heliyon 2019, 5, e02946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.R.; Butz, J.K.; Mengel, M.C. Methods for analysis of gastrointestinal toxicants. Bio. Sci. 2014, 2014, 1–8. [Google Scholar]
- Ren, S.; Liu, M.; Hong, C.; Li, G.; Sun, J.; Wang, J.; Xie, Y. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystal. Acta Pharm. Sin. B 2019, 9, 59–73. [Google Scholar] [CrossRef] [PubMed]





















| Crystal name | Diclofenac-Sodium-Proline-Tetrahydrate (NDPT) |
| Moiety formula | C19H27Cl2N2NaO8, (C14H10Cl2NO2Na) (C5H9NO2) 4(H2O) |
| Crystal system | Monoclinic |
| Space group | P21 |
| a (+) | 7.6073(3) |
| b (Å) | 15.2652(7) |
| c (Å) | 20.5263(9) |
| Β (°) | 97.602(1) |
| V (Å3) | 2362.71(18) |
| Z/Z’ | 4/2 |
| T (K) | 296 |
| R-factor (%) | 5.93 |
| Page: 17 Crystal Name | Diclofenac-Sodium-Proline-Monohydrate (NDPM) |
| Crystal System | Monoclinic |
| Moiety formula | C19H21Cl2N2NaO5, (C14H10Cl2NO2Na) (C5H9NO2) (H2O) |
| Space group | P21 |
| a/Å | 9.8240(3) |
| b/Å | 9.2835(3) |
| c/Å | 21.7931(6) |
| β/° | 100.383(2) |
| V/Å3 | 1955.01(10) |
| Z/Z’ | 4/2 |
| R/% | 7.43 |
| Sample | Solubility (mg/mL) n = 3 | SD | Solubility Magnitude of ND | pH of Solution |
|---|---|---|---|---|
| ND | 16.18 | 0.46 | 1.00 | 6.9–7.0 |
| NDPM | 66.09 | 1.65 | 4.08 | 7.0–7.1 |
| NDPT | 53.61 | 0.83 | 3.31 | 7.0–7.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. https://doi.org/10.3390/pharmaceutics12070690
Nugrahani I, Kumalasari RA, Auli WN, Horikawa A, Uekusa H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics. 2020; 12(7):690. https://doi.org/10.3390/pharmaceutics12070690
Chicago/Turabian StyleNugrahani, Ilma, Rizka A. Kumalasari, Winni N. Auli, Ayano Horikawa, and Hidehiro Uekusa. 2020. "Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study" Pharmaceutics 12, no. 7: 690. https://doi.org/10.3390/pharmaceutics12070690
APA StyleNugrahani, I., Kumalasari, R. A., Auli, W. N., Horikawa, A., & Uekusa, H. (2020). Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics, 12(7), 690. https://doi.org/10.3390/pharmaceutics12070690