Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Drug Stock Solution
2.3. Preparation of Basal Medium and Phosphate Buffer
2.4. Preparation of Fecal Slurry
2.5. Experimental Procedure
2.6. HPLC Analysis
2.7. Data Analysis
3. Results and Discussion
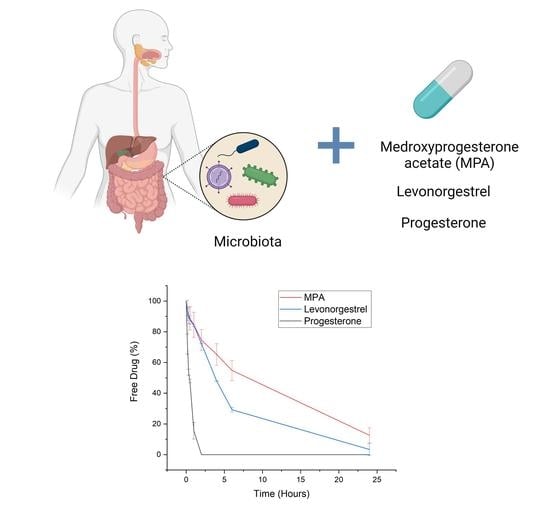
3.1. Degradation of Progesterone by Fecal Microbiota
3.2. Degradation of LevonorGestrel by Fecal Microbiota
3.3. Degradation of Medroxyprogesterone Acetate by Fecal Microbiota
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, T.; Miyagawa, S.; Iguchi, T. Subchapter 94A—Progesterone. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, NY, USA, 2016; pp. 507–508. [Google Scholar]
- Schumacher, M.; Zhu, X.; Guennoun, R. 3.11—Progesterone: Synthesis, Metabolism, Mechanism of Action, and Effects in the Nervous System. In Hormones, Brain and Behavior, 3rd ed.; Pfaff, D.W., Joëls, M., Eds.; Academic Press: Oxford, UK, 2017; pp. 215–244. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Progesterone. In British National Formulary (BNF); NICE: London, UK, 2020. [Google Scholar]
- BesinsHealthcare. Utrogestan 100mg Capsules Summary of Product Characteristics. In Electronic Medicines Compendium; EMC: London, UK, 2019. [Google Scholar]
- Bayer. Levonelle 1500 Microgram Tablet Summary of Product Characteristics. In Electronic Medicines Compendium; EMC: Leverkusen, Germany, 2019. [Google Scholar]
- Pfizer. Provera 10mg Tablets Summary of Product Characteristics. In Electronic Medicines Compendium; EMC: Sandwich, UK, 2020. [Google Scholar]
- Stanczyk, F.Z.; Bhavnani, B.R. Reprint of “Use of medroxyprogesterone acetate for hormone therapy in postmenopausal women: Is it safe?”. J. Steroid Biochem. Mol. Biol. 2015, 153, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B. Drug metabolism by cytochromes P450 in the liver and small bowel. Gastroenterol. Clin. North Am. 1992, 21, 511–526. [Google Scholar] [PubMed]
- Pereira de Sousa, I.; Bernkop-Schnürch, A. Pre-systemic metabolism of orally administered drugs and strategies to overcome it. J. Control. Release 2014, 192, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Tubic-Grozdanis, M.; Hilfinger, J.M.; Amidon, G.L.; Kim, J.S.; Kijek, P.; Staubach, P.; Langguth, P. Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm. Res. 2008, 25, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.; Cristina Freire, A.; Bravo, R.; Basit, A.W. OPTICORE, an innovative and accurate colonic targeting technology. Int. J. Pharm. 2020, 119372. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.; Cristina Freire, A.; Fadda, H.M.; Bravo, R.; Basit, A.W. A dual pH and microbiota-triggered coating (Phloral(TM)) for fail-safe colonic drug release. Int. J. Pharm. 2020, 119379. [Google Scholar] [CrossRef]
- Moens, F.; Van den Abbeele, P.; Basit, A.W.; Dodoo, C.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Stapleton, P.; Basit, A.W.; Gaisford, S. Use of a water-based probiotic to treat common gut pathogens. Int. J. Pharm. 2019, 556, 136–141. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-strain Probiotic Supplement Influences Gut Microbiota Composition and Gut Wall Function in Patients with Ulcerative Colitis. Int. J. Pharm. 2020, 119648. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. (Basel) 2018, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Fadda, H.M.; Sousa, T.; Carlsson, A.S.; Abrahamsson, B.; Williams, J.G.; Kumar, D.; Basit, A.W. Drug Solubility in Luminal Fluids from Different Regions of the Small and Large Intestine of Humans. Mol. Pharm. 2010, 7, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Enright, E.F.; Joyce, S.A.; Gahan, C.G.M.; Griffin, B.T. Impact of gut microbiota-mediated bile acid metabolism on the solubilization capacity of bile salt micelles and drug solubility. Mol. Pharm. 2017, 14, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Scheline, R.R. Metabolism of Foreign Compounds by Gastrointestinal Microorganisms. Pharmacol. Rev. 1973, 25, 451. [Google Scholar] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Sandhu, K.V.; Griffin, B.T.; Dinan, T.G.; Cryan, J.F.; Hyland, N.P. Gut Reactions: Breaking Down Xenobiotic-Microbiome Interactions. Pharmacol. Rev. 2019, 71, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Basit, A.W. Gastrointestinal stability of therapeutic anti-TNF alpha IgG1 monoclonal antibodies. Int. J. Pharm. 2016, 502, 181–187. [Google Scholar] [CrossRef]
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. All disease begins in the gut: Influence of gastrointestinal disorders and surgery on oral drug performance. Int. J. Pharm. 2018, 548, 408–422. [Google Scholar] [CrossRef]
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. Gut reaction: Impact of systemic diseases on gastrointestinal physiology and drug absorption. Drug Discov. Today 2019, 24, 417–427. [Google Scholar] [CrossRef]
- Yadav, V.; Gaisford, S.; Merchant, H.A.; Basit, A.W. Colonic bacterial metabolism of corticosteroids. Int. J. Pharm. 2013, 457, 268–274. [Google Scholar] [CrossRef]
- Sousa, T.; Yadav, V.; Zann, V.; Borde, A.; Abrahamsson, B.; Basit, A.W. On the Colonic Bacterial Metabolism of Azo-Bonded Prodrugsof 5-Aminosalicylic Acid. J. Pharm. Sci. 2014, 103, 3171–3175. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Murdan, S.; Basit, A.W. An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J. Pharm. Sci. 2008, 97, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Stability of peptide drugs in the colon. Eur. J. Pharm. Sci. 2015, 78, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.P.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.; Olavarria-Ramirez, L.; Lach, G.; Boehme, M.; Dinan, T.G.; Cryan, J.F.; Griffin, B.T.; Hyland, N.P.; Clarke, G. Impact of host and environmental factors on β-glucuronidase enzymatic activity: Implications for gastrointestinal serotonin. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G816–G826. [Google Scholar] [CrossRef]
- Basit, A.W.; Newton, J.M.; Lacey, L.F. Susceptibility of the H2-receptor antagonists cimetidine, famotidine and nizatidine, to metabolism by the gastrointestinal microflora. Int. J. Pharm. 2002, 237, 23–33. [Google Scholar] [CrossRef]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations––An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef]
- Enright, E.F.; Gahan, C.G.M.; Joyce, S.A.; Griffin, B.T. The impact of the gut microbiota on drug metabolism and clinical outcome. Yale J. Biol. Med. 2016, 89, 375–382. [Google Scholar]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Haage, D.; Löfgren, M.; Johansson, I.M.; Strömberg, J.; Nyberg, S.; Andréen, L.; Ossewaarde, L.; van Wingen, G.A.; Turkmen, S.; et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience 2011, 191, 46–54. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug-gut microbiota interactions: Implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415–4429. [Google Scholar] [CrossRef] [Green Version]
- Bussy, U.; Boujtita, M. Advances in the Electrochemical Simulation of Oxidation Reactions Mediated by Cytochrome P450. Chem. Res. Toxicol. 2014, 27, 1652–1668. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. All progestins are not created equal. Steroids 2003, 68, 879–890. [Google Scholar] [CrossRef]
- Jones, R.C.; Singer, A.C.; Edgren, R.A. The biological activities of norgestrel and its enantiomers. Int. J. Fertil. 1979, 24, 39–43. [Google Scholar]
- Stanczyk, F.Z.; Roy, S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception 1990, 42, 67–96. [Google Scholar] [CrossRef]
- Martin, F.; Järvenpää, P.; Kosunen, K.; Somers, C.; Lindstrom, B.; Adlercreutz, H. Ring-a reduction of medroxyprogesterone acetate [17α-acetoxy-6α-methyl-4-pregnene-3, 20-dione (MPA)] in biological systems. J. Steroid Biochem. 1980, 12, 491–497. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Liu, Y.; Zhao, J.-Y.; Wang, L.-M.; Ge, G.-B.; Gao, Y.; Li, W.; Liu, H.-T.; Liu, H.-X.; Zhang, Y.-Y.; et al. Metabolic Profiling and Cytochrome P450 Reaction Phenotyping of Medroxyprogesterone Acetate. Drug Metab. Dispos. 2008, 36, 2292. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, J.W.; Yang, L.; Li, W. Structure elucidation of major metabolites from medroxyprogesterone acetate by P450. Chem. Pharm. Bull. 2009, 57, 835–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Althouse, S.K.; Nabell, L.; Rugo, H.; Carey, L.; Kimmick, G.; Jones, D.R.; Merino, M.J.; Steeg, P.S. A phase II study of medroxyprogesterone acetate in patients with hormone receptor negative metastatic breast cancer: Translational breast cancer research consortium trial 007. Breast Cancer Res. Treat. 2014, 148, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Hormone | Rate Constant (min−1) | t ½ (Minutes) |
|---|---|---|
| Progesterone | 0.0316 ± 0.0054 | 28.25 ± 0.42 |
| MPA | 0.0029 ± 0.0016 | 644.18 ± 34.95 |
| LNG | 0.0029 ± 0.0002 | 240.38 ± 3.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coombes, Z.; Yadav, V.; McCoubrey, L.E.; Freire, C.; Basit, A.W.; Conlan, R.S.; Gonzalez, D. Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery. Pharmaceutics 2020, 12, 760. https://doi.org/10.3390/pharmaceutics12080760
Coombes Z, Yadav V, McCoubrey LE, Freire C, Basit AW, Conlan RS, Gonzalez D. Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery. Pharmaceutics. 2020; 12(8):760. https://doi.org/10.3390/pharmaceutics12080760
Chicago/Turabian StyleCoombes, Zoe, Vipul Yadav, Laura E. McCoubrey, Cristina Freire, Abdul W. Basit, R. Steven Conlan, and Deyarina Gonzalez. 2020. "Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery" Pharmaceutics 12, no. 8: 760. https://doi.org/10.3390/pharmaceutics12080760
APA StyleCoombes, Z., Yadav, V., McCoubrey, L. E., Freire, C., Basit, A. W., Conlan, R. S., & Gonzalez, D. (2020). Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery. Pharmaceutics, 12(8), 760. https://doi.org/10.3390/pharmaceutics12080760