Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
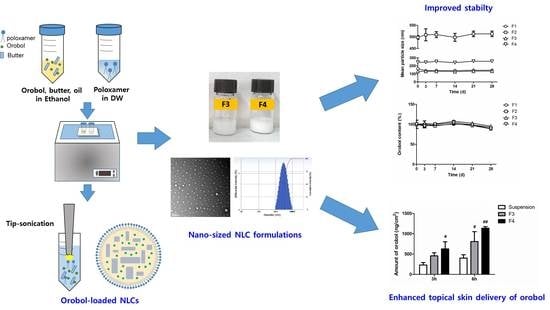
2.2. Preparation of Orobol-Loaded Formulations
2.2.1. Solubility Study
2.2.2. Preparation of Orobol-Loaded NLCs and SLNs
2.3. Characterization of Orobol-Loaded Formulations
2.3.1. Mean Particle Size and Size Distribution
2.3.2. Particle Morphology
2.3.3. Entrapment Efficiency (EE) and Content of Orobol
2.3.4. Powder X-ray Diffraction (pXRD) and Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Study
2.4. Stability Study
2.5. In Vitro Deposition Study Using Artificial Membrane and Human Cadaver Skin
2.6. LC-MS/MS Analysis of Orobol
2.7. Skin Irritation Study in Humans
2.8. Statistical Analysis
3. Results
3.1. Preparation of Orobol-Loaded NLCs and SLNs
3.2. Characterization of Orobol-Loaded SLNs and NLCs
3.3. Stability of Orobol-Loaded SLNs and NLCs
3.4. In Vitro Strat-M Membranes and Human Cadaver Skin Deposition of Orobol
3.5. Skin Irritation of Orobol-Loaded NLCs in Humans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal vitamin D3 as an anti-aging agent for the skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef] [Green Version]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.; Wang, Z.; Li, X.Y.; Quan, T.; Chung, J.H.; Kang, S.; Voorhees, J.J. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Investig. 2000, 106, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, B.Y.; Wu, N.L.; Tsui, W.H.; Lin, T.J.; Su, C.C.; Hung, C.F. Anti-photoaging effects of soy isoflavone extract (aglycone and acetylglucoside form) from soybean cake. Int. J. Mol. Sci. 2010, 12, 4782–4795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campa, M.; Baron, E. Anti-aging effects of select botanicals: Scientific evidence and current trends. Cosmetics 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; Pizzino, G.; D’Anna, R.; Vaccaro, M.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Dietary management of skin health: The role of genistein. Nutrients 2017, 9, 622. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z.D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Kitagawa, S.; Inoue, K.; Teraoka, R.; Morita, S.Y. Enhanced skin delivery of genistein and other two isoflavones by microemulsion and prevention against UV irradiation-induced erythema formation. Chem. Pharm. Bull. 2010, 58, 398–401. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.H.; Kang, M.J.; Lee, J.; Choi, Y.W. Influence of liposome type and skin model on skin permeation and accumulation properties of genistein. J. Dispers. Sci. Technol. 2010, 31, 1061–1066. [Google Scholar] [CrossRef]
- Andrade, L.M.; de Fátima Reis, C.; Maione-Silva, L.; Anjos, J.L.V.; Alonso, A.; Serpa, R.C.; Marreto, R.N.; Lima, E.M.; Taveira, S.F. Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, S.H.; Ji, H.; Kim, J.E.; Yoo, R.; Kim, J.H.; Suk, S.; Huh, C.S.; Park, J.H.Y.; Heo, Y.S.; et al. Orobol, an enzyme-convertible product of genistein, exerts anti-obesity effects by targeting casein kinase 1 epsilon. Sci. Rep. 2019, 9, 8942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.W.; Kwon, J.; Sim, S.J.; Lee, D.; Mar, W. Orobol derivatives and extracts from Cudrania tricuspidata fruits protect against 6-hydroxydomamine-induced neuronal cell death by enhancing proteasome activity and the ubiquitin/proteasome-dependent degradation of α-synuclein and synphilin-1. J. Funct. Food. 2017, 29, 104–114. [Google Scholar] [CrossRef]
- Nam, G.; Ji, Y.; Lee, H.J.; Kang, J.; Yi, Y.; Kim, M.; Lin, Y.; Lee, Y.H.; Lim, M.H. Orobol: An isoflavone exhibiting regulatory multifunctionality against four pathological features of alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Orobol Prevents Solar Ultraviolet-Induced Skin Photoaging by Targeting T-LAK Cell-Originated Protein Kinase. Master’s Thesis, Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea, 31 August 2016. [Google Scholar]
- Kim, K.T.; Kim, M.H.; Park, J.H.; Lee, J.Y.; Cho, H.J.; Yoon, I.S.; Kim, D.D. Microemulsion-based hydrogels for enhancing epidermal/dermal deposition of topically administered 20(S)-protopanaxadiol: In vitro and in vivo evaluation studies. J. Ginseng Res. 2018, 42, 512–523. [Google Scholar] [CrossRef]
- Han, F.; Yin, R.; Che, X.; Yuan, J.; Cui, Y.; Yin, H.; Li, S. Nanostructured lipid carriers (NLC) based topical gel of flurbiprofen: Design, characterization and in vivo evaluation. Int. J. Pharm. 2012, 439, 349–357. [Google Scholar] [CrossRef]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-loaded lipid nanocarriers: Correlation between in vitro occlusion factor and in vivo skin hydrating effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Mirhadi, E.; Nassirli, H.; Malaekeh-Nikouei, B. An updated review on therapeutic effects of nanoparticle-based formulations of saffron components (safranal, crocin, and crocetin). J. Pharm. Investig. 2020, 50, 47–58. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Santana, M.H.; Chorilli, M. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.; Lee, S.E.; Kim, D.H.; Pyo, Y.C.; Park, J.S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Jee, J.P.; Pangeni, R.; Jha, S.K.; Byun, Y.; Park, J.W. Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy. Int. J. Nanomed. 2019, 14, 5449–5475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-M. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.; Yu, M.; Ding, Y.; Zhang, H.; Shen, Y.; Jiang, M.; Liu, P.; Opoku-Damoah, Y.; Webster, T.J.; Zhou, J. Preparation and characterization of nimodipine-loaded nanostructured lipid systems for enhanced solubility and bioavailability. Int. J. Nanomed. 2019, 14, 119–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Lee, J.K.; Jang, W.S.; Kim, T.H.; Tunsirikongkon, A.; Choi, J.S.; Park, J.S. Enhancement of stability and controlled drug release of lipid nanoparticles by modified solvent-evaporation method. Colloids Surf. A Phys. Eng. Asp. 2016, 508, 294–300. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, J.; Kim, M.H.; Park, J.H.; Lee, J.Y.; Song, J.H.; Jung, M.; Jang, M.H.; Cho, H.J.; Yoon, I.S.; et al. Novel reverse electrodialysis-driven iontophoretic system for topical and transdermal delivery of poorly permeable therapeutic agents. Drug Deliv. 2017, 24, 1204–1215. [Google Scholar] [CrossRef] [Green Version]
- Saba, A.M.; Tsado, D.G.; Okafor, J.O. Determination of the effect of storage time and condition on the properties of shea butter. J. Chem. Eng. Process. Technol. 2018, 9, 382. [Google Scholar]
- Iqbal, B.; Ali, J.; Baboota, S. Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. J. Mol. Liq. 2018, 255, 513–529. [Google Scholar] [CrossRef]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak-Kohn, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 441, 56–66. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, K.T.; Sohn, S.Y.; Lee, J.Y.; Lee, C.H.; Yang, H.; Lee, B.K.; Lee, K.W.; Kim, D.D. Formulation and evaluation of nanostructured lipid carriers (NLCs) of 20(S)-protopanaxadiol (PPD) by box-behnken desgin. Int. J. Nanomed. 2019, 14, 8509–8520. [Google Scholar] [CrossRef] [Green Version]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS Pharm. Sci. Technol. 2016, 17, 663–672. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Neelakandan, C.; Kyu, T. Effects of genistein modification on miscibility and hydrogen bonding interactions in poly(amide)/poly(vinyl pyrrolidone) blends and membrane morphology development during coagulation. Polymer 2010, 51, 5135–5144. [Google Scholar] [CrossRef]
- Yatsu, F.K.; Koester, L.S.; Lula, I.; Passos, J.J.; Sinisterra, R.; Bassani, V.L. Multiple comlexation of cyclodextrin with soy isoflavones present in an enriched fraction. Carbohydr. Polym. 2013, 98, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.H.; Michaux, F.; Bouelet Ntsama, I.S.; Durand, P.; Jasniewski, J.; Linder, M. Shea butter solid nanoparticles for curcumin encapsulation: Influence of nanoparticles size on drug loading. Eur. J. Lipid Sci. Technol. 2016, 118, 1168–1178. [Google Scholar]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.W.; Kim, M.H.; Sohn, S.Y.; Kim, K.T.; Park, J.H.; Lee, S.Y.; Lee, J.Y.; Kim, D.D. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018, 182, 245–258. [Google Scholar] [CrossRef]
- Rostagno, M.c.A.; Palma, M.; Barroso, C.G. Short-term stability of soy isoflavones extracts: Sample conservation aspects. Food Chem. 2005, 93, 557–564. [Google Scholar] [CrossRef]
- Yatsu, F.K.J.; Koester, L.S.; Bassani, V.L. Isoflavone-aglycone fraction from glycine max: A promising raw material for isoflavone-based pharmaceutical or nutraceutical products. Braz. J. Pharm. 2016, 26, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.E.; Jung, Y.J.; Choi, Y.J.; Choi, J.S.; Cho, Y.W. Artificial skin models for animal-free testing. J. Pharm. Investig. 2018, 48, 215–223. [Google Scholar] [CrossRef]
- Zsiko, S.; Cutcher, K.; Kovacs, A.; Budai-Szucs, M.; Gacsi, A.; Baki, G.; Csanyi, E.; Berko, S. Nanostructured lipid carrier gel for the dermal application of lidocaine: Comparison of skin penetration testing methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Mbah, C.C.; Builders, P.F.; Agubata, C.O.; Attama, A.A. Development of ethosomal vesicular carrier for topical application of griseofulvin: Effect of ethanol concentration. J. Pharm. Investig. 2019, 49, 27–36. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067–5077. [Google Scholar] [CrossRef] [Green Version]
- Stees, M.; Adjei, I.; Labhasetwar, V. A method for quantification of penetration of nanoparticles through skin layers using near-infrared optical imaging. Cosmetics 2015, 2, 225–235. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Fang, C.L.; Liu, C.H.; Su, Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]






| Phase | Vehicle | Solubility (mg/mL) |
|---|---|---|
| Water | DW | 0.04 ± 0.01 |
| Oil | Capmul MCM EP | 12.37 ± 0.12 |
| Miglyol | 1.03 ± 0.03 | |
| MCT | 1.03 ± 0.05 | |
| Larbrafac CC | 0.89 ± 0.07 | |
| Surfactant | Transcutol | 67.94 ± 1.98 |
| Labrasol | 54.53 ± 3.62 | |
| Tween 20 | 23.81 ± 0.80 |
| Phase | Vehicle | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| Solid lipid | Cocoa butter | 1.5 | 1.5 | - | - |
| Shea butter | - | - | 1.5 | 1.5 | |
| Oil | Capmul MCM EP | - | 0.3 | - | 0.3 |
| Surfactant | Transcutol | 2 | 2 | 2 | 2 |
| Tween 20 | 2 | 2 | 2 | 2 | |
| Water | DW | 94.45 | 94.15 | 94.45 | 94.15 |
| Formulation | Particle Size (nm) | Polydispersity Index (PDI) | Entrapment Efficiency (EE, %) | Loading Content (%) |
| F1 | 165 ± 3 | 0.211 ± 0.001 | 95.7 ± 2.5 | 0.96 ± 0.09 |
| F2 | 498 ± 8 | 0.166 ± 0.042 | 95.9 ± 10.5 | 0.97 ± 0.07 |
| F3 | 133 ± 6 | 0.140 ± 0.016 | 97.2 ± 4.1 | 0.93 ± 0.04 |
| F4 | 246 ± 9 | 0.196 ± 0.016 | 96.8 ± 2.1 | 0.91 ± 0.04 |
| Formulation | 30 Min | 24 H | 48 H |
|---|---|---|---|
| not-treated | 0 | 0 | 0 |
| blank F4 | 0.0167 | 0.0167 | 0.0167 |
| F4 | 0.0167 | 0.0167 | 0.0167 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-H.; Jeon, Y.-E.; Kang, S.; Lee, J.-Y.; Lee, K.W.; Kim, K.-T.; Kim, D.-D. Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol. Pharmaceutics 2020, 12, 845. https://doi.org/10.3390/pharmaceutics12090845
Kim M-H, Jeon Y-E, Kang S, Lee J-Y, Lee KW, Kim K-T, Kim D-D. Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol. Pharmaceutics. 2020; 12(9):845. https://doi.org/10.3390/pharmaceutics12090845
Chicago/Turabian StyleKim, Min-Hwan, Yae-Eun Jeon, Soobeen Kang, Jae-Young Lee, Ki Won Lee, Ki-Taek Kim, and Dae-Duk Kim. 2020. "Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol" Pharmaceutics 12, no. 9: 845. https://doi.org/10.3390/pharmaceutics12090845
APA StyleKim, M. -H., Jeon, Y. -E., Kang, S., Lee, J. -Y., Lee, K. W., Kim, K. -T., & Kim, D. -D. (2020). Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol. Pharmaceutics, 12(9), 845. https://doi.org/10.3390/pharmaceutics12090845