Phytol-Loaded Solid Lipid Nanoparticles as a Novel Anticandidal Nanobiotechnological Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
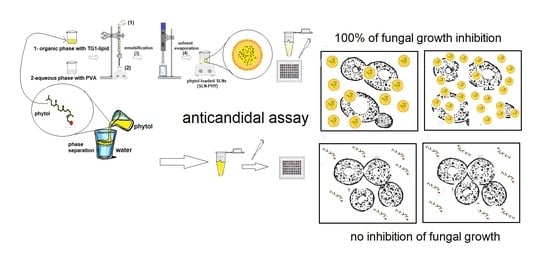
2.2. Preparation of Nanoparticles
2.3. Physicochemical Properties and Stability
2.3.1. Particle Size and Zeta Potential Measurements
2.3.2. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.3. Physicochemical Stability
2.3.4. Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM)
2.4. Drug-Loading Efficiency
2.5. Antifungal Activity
2.5.1. Candida spp. Strains and Clinical Isolates
2.5.2. Fungal Minimal Inhibitory Concentration (MIC) Determination of Phytol and Nanoparticles
2.6. Cell Viability Experiments
2.6.1. Cell Culture
2.6.2. In Vitro Cell Viability Assay
2.7. Statistical
3. Results and Discussion
3.1. Preparation of Drug-Loaded Solid Lipid Nanoparticles
3.2. Morphology and Physicochemical Stability
3.3. Mean Diameter and Zeta Potential as a Function of Storage Time for Different Formulations of SLN
3.4. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.5. Anticandidal Assays
3.5.1. Minimal Inhibitory Concentration (MIC) of Growth in Reference Strains of Candida spp.
3.5.2. Fungal Minimal Inhibitory Concentration (MIC) in Candida spp. Clinical Isolates
3.6. In Vitro Viability Assay
4. Conclusions
5. Patients
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Metin, A.; Dilek, N.; Bilgili, S.G. Recurrent candidal intertrigo: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2018, 11, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans–epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, civ933. [Google Scholar] [CrossRef]
- De Medeiros, M.A.P.; Vieira De Melo, P.; Gonçalves, S.S.; Milan, E.P.; Chaves, G.M. Genetic relatedness among vaginal and anal isolates of Candida albicans from women with vulvovaginal candidiasis in North-East Brazil. J. Med. Microbiol. 2014, 63, 1436–1445. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.; Ostrosky-Zeichner, L.; et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [Green Version]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global Burden of Fungal Diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef]
- de Cássia Orlandi Sardi, J.; Silva, D.R.; Soares Mendes-Giannini, M.J.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Souza, M.E.; Lopes, L.Q.S.; Bonez, P.C.; Gündel, A.; Martinez, D.S.T.; Sagrillo, M.R.; Giongo, J.L.; Vaucher, R.A.; Raffin, R.P.; Boligon, A.A.; et al. Melaleuca alternifolia nanoparticles against Candida species biofilms. Microb. Pathog. 2017, 104, 125–132. [Google Scholar] [CrossRef]
- Siqueira, A.B.S.; Rodriguez, L.R.N.D.A.; SANTOS, R.K.B.; Marinho, R.R.B.; Abreu, S.; Peixoto, R.F.; Gurgel, B.C.D.V. Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- de Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.G.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L.S. Phytol, a Diterpene Alcohol from Chlorophyll, as a Drug against Neglected Tropical Disease Schistosomiasis Mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef] [Green Version]
- Baldim, I.; Tonani, L.; von Zeska Kress, M.R.; Pereira Oliveira, W. Lippia sidoides essential oil encapsulated in lipid nanosystem as an anti-Candida agent. Ind. Crops Prod. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Islam, M.T.; da Mata, A.M.O.F.; de Aguiar, R.P.S.; Paz, M.F.C.J.; de Alencar, M.V.O.B.; Ferreira, P.M.P.; de Carvalho Melo-Cavalcante, A.A. Therapeutic Potential of Essential Oils Focusing on Diterpenes. Phyther. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Islam, M.T.; de Alencar, M.V.O.B.; da Conceição Machado, K.; da Conceição Machado, K.; de Carvalho Melo-Cavalcante, A.A.; de Sousa, D.P.; de Freitas, R.M. Phytol in a pharma-medico-stance. Chem. Biol. Interact. 2015, 240, 60–73. [Google Scholar] [CrossRef]
- Islam, M.T. Diterpenes and Their Derivatives as Potential Anticancer Agents. Phyther. Res. 2017, 31, 691–712. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Ehrampoush, M.H.; Jebali, A.; Hekmatimoghaddam, S.; Mahmoudi, M. Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ. Heal. Eng. Manag. J. 2015, 2, 13–16. [Google Scholar]
- Costa, J.P.; Islam, M.T.; Santos, P.S.; Ferreira, P.B.; Oliveira, G.L.S.; Alencar, M.V.O.B.; Paz, M.F.C.J.; Ferreira, É.L.F.; Feitosa, C.M.; Citó, A.M.G.L.; et al. Evaluation of Antioxidant Activity of Phytol Using Non- and Pre-Clinical Models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef]
- Islam, M.T.; Streck, L.; Correia Jardim Paz, M.F.; de Castro e Sousa, J.M.; Oliveira Barros de Alencar, M.V.; Oliveira Ferreira da Mata, A.M.; Melo de Carvalho, R.; de Oliveira Santos, J.V.; da Silva-Junior, A.A.; Pinheiro Ferreira, P.M.; et al. Preparation of phytol-loaded nanoemulsion and screening for antioxidant capacity. Int. Arch. Med. 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, R.; Malar, D.S.; Devi, K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef]
- Pejin, B.; Savic, A.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Radotic, K.; Mojovic, M. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat. Prod. Res. 2014, 28, 372–376. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on phytol. Food Chem. Toxicol. 2010, 48, S59–S63. [Google Scholar] [CrossRef]
- Nakhlband, A.; Eskandani, M.; Saeedi, N.; Ghafari, S.; Omidi, Y.; Barar, J.; Garjani, A. Marrubiin-loaded solid lipid nanoparticles’ impact on TNF-α treated umbilical vein endothelial cells: A study for cardioprotective effect. Colloids Surf. B Biointerfaces 2018, 164, 299–307. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Saranya, S.; Vaidevi, S.; Ruckmani, K.; Pandima Devi, K. Phytol-loaded PLGA nanoparticle as a modulator of Alzheimer’s toxic Aβ peptide aggregation and fibrillation associated with impaired neuronal cell function. Artif. Cells Nanomedicine Biotechnol. 2017, 46, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Sathya, S.; Shanmuganathan, B.; Balasubramaniam, B.; Balamurugan, K.; Devi, K.P. Phytol loaded PLGA nanoparticles regulate the expression of Alzheimer’s related genes and neuronal apoptosis against amyloid-β induced toxicity in Neuro-2a cells and transgenic Caenorhabditis elegans. Food Chem. Toxicol. 2020, 136, 110962. [Google Scholar] [CrossRef]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Streck, L.; de Alencar, M.V.O.B.; Cardoso Silva, S.W.; da Conceição Machado, K.; da Conceição Machado, K.; Gomes Júnior, A.L.; Paz, M.F.C.J.; da Mata, A.M.O.F.; de Castro e Sousa, J.M.; et al. Evaluation of toxic, cytotoxic and genotoxic effects of phytol and its nanoemulsion. Chemosphere 2017, 177, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, B.E.; Afolayan, A.J.; Bradley, G. Chemical composition profiling and antifungal activity of the essential oil and plant extracts of Mesembryanthemum edule (L.) bolus leaves. Afr J Tradit Complement Altern Med. 2014, 11, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakadia, P.G.; Conway, B.R. Solid Lipid Nanoparticles: A Potential Approach for Dermal Drug Delivery. Am. J. Pharmacol. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Reference Method for Broth Dilution; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 3. [Google Scholar]
- Souza, L.; Silva-Rocha, W.; Ferreira, M.; Soares, L.; Svidzinski, T.; Milan, E.; Pires, R.; Fusco Almeida, A.; Mendes-Giannini, M.; Maranhão Chaves, G. Influence of Eugenia uniflora Extract on Adhesion to Human Buccal Epithelial Cells, Biofilm Formation, and Cell Surface Hydrophobicity of Candida spp. from the Oral Cavity of Kidney Transplant Recipients. Molecules 2018, 23, 2418. [Google Scholar] [CrossRef] [Green Version]
- Lima, T.; Feitosa, R.; dos Santos-Silva, E.; dos Santos-Silva, A.; Siqueira, E.; Machado, P.; Cornélio, A.; do Egito, E.; Fernandes-Pedrosa, M.; Farias, K.; et al. Improving Encapsulation of Hydrophilic Chloroquine Diphosphate into Biodegradable Nanoparticles: A Promising Approach against Herpes Virus Simplex-1 Infection. Pharmaceutics 2018, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- dos Santos-Silva, A.M.; de Caland, L.B.; de SL Oliveira, A.L.C.; de Araújo-Júnior, R.F.; Fernandes-Pedrosa, M.F.; Cornélio, A.M.; da Silva-Júnior, A.A. Designing structural features of novel benznidazole-loaded cationic nanoparticles for inducing slow drug release and improvement of biological efficacy. Mater. Sci. Eng. C 2017, 78, 978–987. [Google Scholar] [CrossRef]
- Pooja, D.; Tunki, L.; Kulhari, H.; Reddy, B.B.; Sistla, R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data Br. 2016, 6, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadi, S.; Serpooshan, V.; Wei, T.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey Inside the Cell. Chem Soc Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Bahadori, M.B.; Vandghanooni, S.; Eskandani, M.; Nakhlband, A.; Eskandani, M. Preparation, characterization and anti-proliferative effects of sclareol-loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J. Drug Deliv. Sci. Technol. 2018, 45, 272–280. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, W.; Duan, C.; Jia, L.; Wang, Y.; Feng, F.; Zhang, Q. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J. Microencapsul. 2010, 27, 234–241. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Dendisová, M.; Jeništová, A.; Parchaňská-kokaislová, A.; Matějka, P.; Prokopec, V.; Švecová, M. The use of infrared spectroscopic techniques to characterize nanomaterials and nanostructures: A review. Anal. Chim. Acta 2018, 1031, 1–14. [Google Scholar] [CrossRef]
- Matos, S.; Carvalho, D.; Montanheiro, C.; Gonçalves, C.; Gustavo, W.; Casagrande, I.; Ramos, M.; Cleber, F.; Luiz, P.; Barreto, M. PVA antioxidant nanocomposite films functionalized with alpha-tocopherol loaded solid lipid nanoparticles. Colloids Surf. A 2019, 581, 123793. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Feitosa, C.M.; dos Santos, P.R.P.; de Freitas, R.M.; Rodrigues, A.M.X.; de Oliveira, G.A.L.; da Costa Junior, J.S.; Cavalcante, A.D.N. Pre clinical trials in rats treated with 1,3-distearoyl-2-oleoylglycerol (TG1) constituent isolated from Platonia insignis. ConScientiae Saúde 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Sequeda, N.; Torres, R.; Ortiz, C. Synthesis, characterization, and in vitro activity against Candida spp. Of fluconazole encapsulated on cationic and conventional nanoparticles of poly(lactic-co-glycolic acid). Nanotechnol. Sci. Appl. 2017, 10, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues Bianchin, M.; Borowicz, S.M.; da Rosa Monte Machado, G.; Pippi, B.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Meneghello Fuentefria, A.; Clemes Külkamp-Guerreiro, I. Lipid core nanoparticles as a broad strategy to reverse fluconazole resistance in multiple Candida species. Colloids Surf. B Biointerfaces 2019, 175, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Kelidari, H.R.; Saeedi, M.; Morteza-Semnani, K.; Nabili, M.; Abdollahi Gohar, A.; Akbari, J.; Lotfali, E.; Nokhodchi, A. Time to overcome fluconazole resistant Candida isolates: Solid lipid nanoparticles as a novel antifungal drug delivery system. Colloids Surf. B Biointerfaces 2016, 142, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Svetlichny, G.; Kulkamp-Guerreiro, I.C.; Cunha, S.L.; Silva, F.E.K.; Bueno, K.; Pohlmann, A.R.; Fuentefria, A.M.; Guterres, S.S. Solid lipid nanoparticles containing copaiba oil and allantoin: Development and role of nanoencapsulation on the antifungal activity. Die Pharm. Int. J. Pharm. Sci. 2015, 70, 155–164. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; de Oliveira, A.L.G.; Lacerda, J.S.; Rezende-Júnior, L.M.; da Silva, M.L.G.; Coêlho, M.L.; Tomé, A.R.; da Costa-Júnior, J.S.; Ferraz, A.B.F.; David, J.M.; et al. Evaluation of the cicatrizant activity of a semisolid pharmaceutical formulation obtained from Platonia insignis Mart. Afr. J. Pharm. Pharmacol. 2015, 9, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Diekema, D.; Arbefeville, S.; Boyken, L.; Kroeger, J.; Pfaller, M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 2012, 73, 45–48. [Google Scholar] [CrossRef]
- Vieira de Melo, A.P.; Zuza-Alves, D.L.; da Silva-Rocha, W.P.; Ferreira Canário de Souza, L.B.; Francisco, E.C.; Salles de Azevedo Melo, A.; Maranhão Chaves, G. Virulence factors of Candida spp. obtained from blood cultures of patients with candidemia attended at tertiary hospitals in Northeast Brazil. J. Mycol. Med. 2019. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, R.; Li, Y.; Wang, Z.; Ishchuk, O.P.; Ahmad, K.M.; Wee, J.; Piskur, J.; Shapiro, J.A.; Gu, Z. Understand the genomic diversity and evolution of fungal pathogen Candida glabrata by genome-wide analysis of genetic variations. Methods 2019. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre, J.M.; Quindós, G.; Eraso, E. Prevalence and antifungal susceptibility profiles of Candida glabrata, Candida parapsilosis and their close-related species in oral candidiasis. Arch. Oral Biol. 2018, 95, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.P.D.; Melo, A.P.V.D.; Bento, A.D.O.; Souza, L.B.F.C.D.; Neto, F.D.A.B.; Garcia, J.B.L.; Luzia-Alves, D.; Francisco, E.C.; Melo, A.S.D.A.D.; Chaves, G.M. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE 2019, 14, e0221033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shan, Y.; Fan, S.; Li, J.; Liu, X. Candida parapsilosis Sensu Stricto and the Closely Related Species Candida orthopsilosis and Candida metapsilosis in Vulvovaginal Candidiasis. Mycopathologia 2015, 179, 111–118. [Google Scholar] [CrossRef]
- Hashemi, S.E.; Shokohi, T.; Abastabar, M.; Aslani, N.; Ghadamzadeh, M.; Haghani, I. Species distribution and susceptibility profiles of Candida species isolated from vulvovaginal candidiasis, emergence of C. lusitaniae. Curr. Med Mycol. 2019, 5, 26–34. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Kumar, P.; Bhatt, R.P.; Manzoor, N. Antifungal activity of Coriaria nepalensis essential oil by disrupting ergosterol biosynthesis and membrane integrity against Candida. Yeast 2011, 28, 611–617. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Barros de Alencar, M.V.O.; de Castro e Sousa, J.M.; Rolim, H.M.L.; de Medeiros, M.d.G.F.; Cerqueira, G.S.; de Castro Almeida, F.R.; Citó, A.M.d.G.L.; Ferreira, P.M.P.; Lopes, J.A.D.; de Carvalho Melo-Cavalcante, A.A.; et al. Diterpenes as lead molecules against neglected tropical diseases. Phyther. Res. 2017, 31, 175–201. [Google Scholar] [CrossRef]
- K Mazu, T.; A Bricker, B.; Flores-Rozas, H.; Y Ablordeppey, S. The Mechanistic Targets of Antifungal Agents: An Overview. Mini-Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials 2008, 29, 4244–4252. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Latge, J.-P.; Aimanianda, V. Undressing the Fungal Cell Wall/Cell Membrane-the Antifungal Drug Targets. Curr. Pharm. Des. 2013, 19, 3738–3747. [Google Scholar] [CrossRef] [PubMed]




| Nanoparticles | TG1/Phytol Ratio | pH | Size (nm) | PdI | ZP (mV) | EE (%) |
|---|---|---|---|---|---|---|
| SLN-B | - | 6.8 | 297.6 ± 28.5 | 0.15 ± 0.03 | −16.0 ± 2.2 | - |
| 10-SLN-PHY | 1:10 | 6.4 | 307.4 ± 12.5 | 0.15 ± 0.07 | −16.3 ± 11.1 | 67.38 |
| 5-SLN-PHY | 1:5 | 6.5 | 302.2 ± 10.0 | 0.12 ± 0.03 | −16.7 ± 0.5 | 68.74 |
| 3-SLN-PHY | 1:3 | 6.5 | 297.9 ± 2.4 | 0.14 ± 0.02 | −18.6 ± 1.0 | 68.20 |
| Candida spp. Reference Strains | MIC (µg/mL) of Formulations | |||||
|---|---|---|---|---|---|---|
| MIC 50 | MIC 100 | |||||
| FLU | PHY | SLN-B | 10-SLN-PHY | 5-SLN-PHY | 3-SLN-PHY | |
| C. albicans ATCC 90028 | 0.125 | 2500 | >173.5 | 7.81 | 0.24 | 0.40 |
| C. dubliniensis CBS 7987 | 0.5 | 2500 | >173.5 | 7.81 | 0.24 | 0.40 |
| C. tropicalis ATCC 13803 | 0.5 | 1250 | >173.5 | 7.81 | 0.24 | 0.40 |
| C. parapsilosis ATCC 22019 | 0.5 | 1250 | >173.5 | 3.90 | 0.24 | 0.40 |
| C. glabrata ATCC 2001 | 0.5 | 625 | >173.5 | 1.95 | 0.24 | 0.40 |
| C. rugosa ATCC 10571 | 0.125 | 1250 | >173.5 | 15.62 | 0.24 | 0.40 |
| C. krusei ATCC 6258 | 16.0 | 2500 | >173.5 | 15.62 | 0.24 | 0.40 |
| Candida spp. Clinical Isolates | MIC (µg/mL) of Formulations | |||||
|---|---|---|---|---|---|---|
| MIC 50 | MIC 100 | |||||
| FLU | PHY | SLN-B | 10-SLN-PHY | 5-SLN-PHY | 3-SLN-PHY | |
| C. albicans LMMM 92 | 0.5 | >10,000 | >173.5 | >62.5 | >125 | >208.3 |
| C. albicans LMMM 100 | 0.5 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. tropicalis LMMM 195 | 1.0 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. tropicalis LMMM 447 | 2.0 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. parapsilosis LMMM 83 | 0.125 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. parapsilosis LMMM 85 | 16.0 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. glabrata LMMM 704 | 2.0 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| C. krusei LMMM 249 | 16.0 | >10,000 | >173.5 | >62.5 | 0.24 | 0.40 |
| Formulations | (CC50 µg/mL) |
|---|---|
| PHY | >250 |
| SLN-B | >347 |
| 5-SLN-PHY | 13.34 |
| 3-SLN-PHY | 10.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, T.L.C.; Souza, L.B.F.C.; Tavares-Pessoa, L.C.S.; Santos-Silva, A.M.d.; Cavalcante, R.S.; Araújo-Júnior, R.F.d.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; Chaves, G.M.; Silva-Júnior, A.A.d. Phytol-Loaded Solid Lipid Nanoparticles as a Novel Anticandidal Nanobiotechnological Approach. Pharmaceutics 2020, 12, 871. https://doi.org/10.3390/pharmaceutics12090871
Lima TLC, Souza LBFC, Tavares-Pessoa LCS, Santos-Silva AMd, Cavalcante RS, Araújo-Júnior RFd, Cornélio AM, Fernandes-Pedrosa MF, Chaves GM, Silva-Júnior AAd. Phytol-Loaded Solid Lipid Nanoparticles as a Novel Anticandidal Nanobiotechnological Approach. Pharmaceutics. 2020; 12(9):871. https://doi.org/10.3390/pharmaceutics12090871
Chicago/Turabian StyleLima, Tábata L. C., Luanda B. F. C. Souza, Lannya C. S. Tavares-Pessoa, Alaine M. dos Santos-Silva, Rômulo S. Cavalcante, Raimundo F. de Araújo-Júnior, Alianda M. Cornélio, Matheus F. Fernandes-Pedrosa, Guilherme Maranhão Chaves, and Arnóbio Antônio da Silva-Júnior. 2020. "Phytol-Loaded Solid Lipid Nanoparticles as a Novel Anticandidal Nanobiotechnological Approach" Pharmaceutics 12, no. 9: 871. https://doi.org/10.3390/pharmaceutics12090871
APA StyleLima, T. L. C., Souza, L. B. F. C., Tavares-Pessoa, L. C. S., Santos-Silva, A. M. d., Cavalcante, R. S., Araújo-Júnior, R. F. d., Cornélio, A. M., Fernandes-Pedrosa, M. F., Chaves, G. M., & Silva-Júnior, A. A. d. (2020). Phytol-Loaded Solid Lipid Nanoparticles as a Novel Anticandidal Nanobiotechnological Approach. Pharmaceutics, 12(9), 871. https://doi.org/10.3390/pharmaceutics12090871