Therapeutic Ophthalmic Lenses: A Review
Abstract
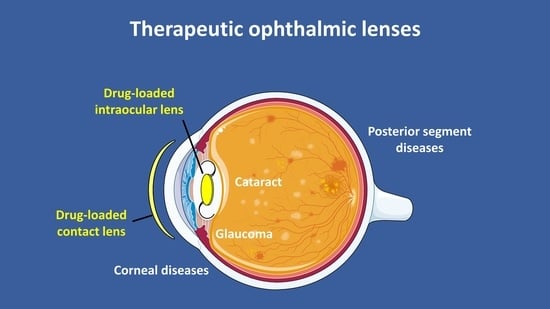
:1. Introduction
2. Methodology
3. Drug Loading Methods
3.1. Soaking
3.2. Incorporation of Functional Molecules
3.3. Coating
3.4. Molecular Imprinting
3.5. Supercritical Impregnation
3.6. Incorporation of Nanocarriers
3.7. Drug Reservoirs
4. Lenses for Ocular Diseases
4.1. Glaucoma
4.2. Cataract
4.3. Corneal Diseases
4.4. Posterior Segment Diseases
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Medical terms | |
| AGEs | Advanced glycation end products |
| AMD | Age-related macular degeneration |
| CLs | Contact lenses |
| DME | Diabetic macular edema |
| DR | Diabetic retinopathy |
| IOLs | Intraocular lenses |
| IOP | Intraocular pressure |
| PCME | Pseudophakic cystoid macular edema |
| PCO | Posterior capsule opacification |
Therapeutics/drugs | |
| ARI(s) | Aldose reductase inhibitor(s) |
| EGF | Epidermal growth factor |
| LF | Lactoferrin |
| MMPI | Matrix metalloproteinases inhibitors |
| MTX | Methotrexate |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| NTX | Naltrexone |
| PDGF | Platelet-derived growth factor |
| TM | Timolol maleate |
| anti-VEGF | Vascular endothelial growth factor inhibitor |
Materials and processing terms | |
| (p)HEMA | (poly)hydroxyethyl methacrylate |
| PBS | Phosphate buffered saline |
| Sil | Siloxane macromers |
| PGT | Propoxylated glyceryl triacylate |
| EGDMA | Ethylene glycol dimethacrylate |
| PEG | Polyethylene glycol |
| PLA | Polylactide |
| PNIPAM | Poly-n-isopropylacrylamide |
| MAA | Methacrylic acid |
| MA | Methyl acrylate |
| DMA | N,N-dimethylacrylamide |
| PLGA | Poly(lactic-co-glycolic acid) |
| PDMS | Poly(dimethyl)siloxane |
| APMA | Aminopropyl methacrylamide |
| MMA | Methyl methacrylate |
| BEM | 2-butoxyethyl methacrylate |
| PVA | Polyvinyl alcohol |
| HA | Hyaluronic acid |
| HPMC | Hydroxypropyl methylcellulose |
| LbL | Layer-by-layer |
| NVP | N-vinylpyrrolidone |
| PVP | Polyvinylpyrrolidone |
| GNP | Gold nanoparticles |
| GMA | Glycidyl methacrylate |
| PC | Phosphorylcholine |
| IBM | Isobornyl methacrylate |
| HBM | 2-hydroxybutyl methacrylate |
| MVA | N-methyl-N-vinylacetamide |
| PEA | Phenylethyl acrylate |
| PEMA | Phenylethyl methacrylate |
| BMA | Benzyl methacrylate |
| EGPEM | Ethyleneglycolphenylether methacrylate |
References
- Shatz, W.; Aaronson, J.; Yohe, S.; Kelley, R.F.; Kalia, Y.N. Strategies for modifying drug residence time and ocular bioavailability to decrease treatment frequency for back of the eye diseases. Expert Opin. Drug Deliv. 2019, 16, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, M.; Barikani, A.; Ghasemi, H. Eye disorders in old people. Glob. J. Health Sci. 2013, 5, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balyeat, R.M.; Bowen, R. Allergic conjunctivitis. Community Eye Health J. 2017, 29, S7–S10. [Google Scholar] [CrossRef]
- Priyavarshini, R.; Amit, B.P. Recent development in the artificial treatment for dry eye disease. Int. J. Res. Pharm. Sci. 2020, 11, 1902–1907. [Google Scholar] [CrossRef]
- Song, B.J.; Aiello, L.P.; Pasquale, L.R. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr. Diabetes Rep. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef] [Green Version]
- Shah, T.J.; Conway, M.D.; Peyman, G.A. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: Design, development, and place in therapy. Clin. Ophthalmol. 2018, 12, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Tyson, S.L.; Bailey, R.; Roman, J.S.; Zhan, T.; Hark, L.A.; Haller, J.A. Clinical outcomes after injection of a compounded pharmaceutical for prophylaxis after cataract surgery: A large-scale review. Curr. Opin. Ophthalmol. 2017, 28, 73–80. [Google Scholar] [CrossRef]
- Hermann, M.M.; Stündag, C.Ü.; Diestelhorst, M. Electronic compliance monitoring of topical treatment after ophthalmic surgery. Int. Ophthalmol. 2010, 30, 385–390. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- An, J.A.; Kasner, O.; Samek, D.A.; Lévesque, V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J. Cataract Refract. Surg. 2014, 40, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, A.G.M.; Chorągiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A nanoparticle-based ophthalmic formulation of dexamethasone enhances corneal permeability of the drug and prolongs its corneal residence time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.T.W.; Herceg, M.C.; Bilonick, R.A.; Camejo, L.; Schuman, J.S.; Noecker, R.J. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin. Ophthalmol. 2009, 3, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiernan, D.F.; Hariprasad, S.M. Controversies in the management of Irvine-Gass syndrome. Ophthalmic Surg. Lasers Imaging 2013, 44, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzybowski, A.; Kanclerz, P. The Role of Steroids and NSAIDs in Prevention and Treatment of Postsurgical Cystoid Macular Edema. Curr. Pharm. Des. 2019, 24, 4896–4902. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Hui, A. Contact lenses for ophthalmic drug delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef] [Green Version]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Garty, S.; Shirakawa, R.; Warsen, A.; Anderson, E.M.; Noble, M.L.; Bryers, J.D.; Ratner, B.D.; Shen, T.T. Sustained antibiotic release from an intraocular lens-hydrogel assembly for cataract surgery. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6109–6116. [Google Scholar] [CrossRef] [Green Version]
- Topete, A.; Serro, A.P.; Saramago, B. Dual drug delivery from intraocular lens material for prophylaxis of endophthalmitis in cataract surgery. Int. J. Pharm. 2019, 558, 43–52. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [Green Version]
- Bouledjouidja, A.; Masmoudi, Y.; Sergent, M.; Trivedi, V.; Meniai, A.; Badens, E. Drug loading of foldable commercial intraocular lenses using supercritical impregnation. Int. J. Pharm. 2016, 500, 85–99. [Google Scholar] [CrossRef]
- Eperon, S.; Bossy-Nobs, L.; Petropoulos, I.K.; Gurny, R.; Guex-Crosier, Y. A biodegradable drug delivery system for the treatment of postoperative inflammation. Int. J. Pharm. 2008, 352, 240–247. [Google Scholar] [CrossRef]
- Fonn, D.; Sweeney, D. The Benefits of Silicone Hydrogel Daily Disposable Lenses: A look at why silicone hydrogel daily disposable lenses are our preferred option for daily wear. Contact Lens Spectr. 2015, 30, 42–45. [Google Scholar]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From Fundamentals to Applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef]
- Mohammadi, S.; Jones, L.; Gorbet, M. Extended latanoprost release from commercial contact lenses: in vitro studies using corneal models. PLoS ONE 2014, 9, e106653. [Google Scholar] [CrossRef] [Green Version]
- Schultz, C.; Breaux, J.; Schentag, J.; Morck, D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin. Exp. Optom. 2011, 94, 212–218. [Google Scholar] [CrossRef]
- Davis, J.L.; Yi, N.Y.; Salmon, J.H.; Charlton, A.N.; Colitz, C.M.H.; Gilger, B.C. Sustained-release celecoxib from incubated acrylic intraocular lenses suppresses lens epithelial cell growth in an ex vivo model of posterior capsule opacity. J. Ocul. Pharmacol. Ther. 2012, 28, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Wertheimer, C.; Brandlhuber, U.; Kook, D.; Mayer, W.J.; Laubichler, P.; Wolf, A.; Kampik, A.; Eibl-Lindner, K. Erufosine, a phosphoinositide-3-kinase inhibitor, to mitigate posterior capsule opacification in the human capsular bag model. J. Cataract Refract. Surg. 2015, 41, 1484–1489. [Google Scholar] [CrossRef]
- Wertheimer, C.; Kueres, A.; Siedlecki, J.; Braun, C.; Kassumeh, S.; Wolf, A.; Mayer, W.; Priglinger, C.; Priglinger, S.; Eibl-Lindner, K. The intraocular lens as a drug delivery device for an epidermal growth factor–Receptor inhibitor for prophylaxis of posterior capsule opacification. Acta Ophthalmol. 2018, 96, e874–e882. [Google Scholar] [CrossRef] [Green Version]
- Kassumeh, S.; Kueres, A.; Hillenmayer, A.; von Studnitz, A.; Elhardt, C.; Ohlmann, A.; Priglinger, S.G.; Wertheimer, C.M. Development of a drug-eluting intraocular lens to deliver epidermal growth factor receptor inhibitor gefitinib for posterior capsule opacification prophylaxis. Eur. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Wertheimer, C.; Kassumeh, S.; Piravej, N.P.; Nilmayer, O.; Braun, C.; Priglinger, C.; Luft, N.; Wolf, A.; Mayer, W.J.; Priglinger, S.G.; et al. The intraocular lens as a drug delivery device: in vitro screening of pharmacologic substances for the prophylaxis of posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6408–6418. [Google Scholar] [CrossRef] [Green Version]
- Atabek Yigit, E.; Ercal, N. Release of N-acetylcysteine and N-acetylcysteine amide from contact lenses. Eye Contact Lens 2013, 39, 335–340. [Google Scholar] [CrossRef]
- Horne, R.R.; Rich, J.T.; Bradley, M.W.; Pitt, W.G. Latanoprost uptake and release from commercial contact lenses. J. Biomater. Sci. Polym. Ed. 2020, 31, 1–19. [Google Scholar] [CrossRef]
- Lipnitzki, I.; Bronshtein, R.; Ben Eliahu, S.; Marcovich, A.L.; Kleinmann, G. Hydrophilic acrylic intraocular lens as a drug delivery system: Influence of the presoaking time and comparison to intracameral injection. J. Ocul. Pharmacol. Ther. 2013, 29, 414–418. [Google Scholar] [CrossRef]
- Topete, A.; Oliveira, A.S.; Fernandes, A.; Nunes, T.G.; Serro, A.P.; Saramago, B. Improving sustained drug delivery from ophthalmic lens materials through the control of temperature and time of loading. Eur. J. Pharm. Sci. 2018, 117, 107–117. [Google Scholar] [CrossRef]
- Filipe, H.P.; Bozukova, D.; Pimenta, A.; Vieira, A.P.; Oliveira, A.S.; Galante, R.; Topete, A.; Masson, M.; Alves, P.; Coimbra, P.; et al. Moxifloxacin-loaded acrylic intraocular lenses: in vitro and in vivo performance. J. Cataract Refract. Surg. 2019, 45, 1808–1817. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Serro, A.P.; Colaço, R.; Chauhan, A. Optimization of intraocular lens hydrogels for dual drug release: Experimentation and modelling. Eur. J. Pharm. Biopharm. 2019, 141, 51–57. [Google Scholar] [CrossRef]
- Schultz, C.L.; Morck, D.W. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin. Exp. Optom. 2010, 93, 61–65. [Google Scholar] [CrossRef]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-loaded contact lenses: Eye protection against oxidative stress. Cornea 2015, 34, 693–697. [Google Scholar] [CrossRef]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-loaded contact lenses counteract cytotoxicity caused in vitro by keratoconic tears. Contact Lens Anterior Eye 2019, 42, 253–257. [Google Scholar] [CrossRef]
- González-Chomón, C.; Concheiro, A.; Alvarez-Lorenzo, C. Drug-eluting intraocular lenses. Materials 2011, 4, 1927–1940. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef]
- Madni, A.; Rahem, M.A.; Tahir, N.; Sarfraz, M.; Jabar, A.; Rehman, M.; Kashif, P.M.; Badshah, S.F.; Khan, K.U.; Santos, H.A. Non-invasive strategies for targeting the posterior segment of eye. Int. J. Pharm. 2017, 530, 326–345. [Google Scholar] [CrossRef]
- Dixon, P.; Chauhan, A. Effect of the surface layer on drug release from delefilcon-A (Dailies Total1®) contact lenses. Int. J. Pharm. 2017, 529, 89–101. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- García-Fernández, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(cyclo)dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses. Carbohydr. Polym. 2013, 98, 1343–1352. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin e loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Sekar, P.; Chauhan, A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef]
- Lee, D.; Cho, S.; Park, H.S.; Kwon, I. Ocular drug delivery through pHEMA-Hydrogel contact lenses Co-loaded with lipophilic vitamins. Sci. Rep. 2016, 6, 34194. [Google Scholar] [CrossRef]
- Peng, C.C.; Ben-Shlomo, A.; MacKay, E.O.; Plummer, C.E.; Chauhan, A. Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr. Eye Res. 2012, 37, 204–211. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Kim, J.; Peng, C.C.; Chauhan, A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Koolivand, A.; Fan, X.; Agrawal, N.R.; Hu, N.; Zhu, Y.; Domszy, R.; Briber, R.M.; Wang, N.S.; Yang, A. Formation of drug-participating catanionic aggregates for extended delivery of non-steroidal anti-inflammatory drugs from contact lenses. Biomolecules 2019, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the Loading and Release of NSAIDs frompHEMA Hydrogels by Copolymerization withFunctionalized Monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar] [CrossRef]
- González-Chomón, C.; Braga, M.E.M.; De Sousa, H.C.; Concheiro, A.; Alvarez-Lorenzo, C. Antifouling foldable acrylic IOLs loaded with norfloxacin by aqueous soaking and by supercritical carbon dioxide technology. Eur. J. Pharm. Biopharm. 2012, 82, 383–391. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Preparation and evaluation of a contact lens vehicle for puerarin delivery. J. Biomater. Sci. Polym. Ed. 2010, 21, 271–288. [Google Scholar] [CrossRef]
- Kakisu, K.; Matsunaga, T.; Kobayakawa, S.; Sato, T.; Tochikubo, T. Development and efficacy of a drug-releasing soft contact lens. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Soluri, A.; Hui, A.; Jones, L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Kassumeh, S.A.; Wertheimer, C.M.; von Studnitz, A.; Hillenmayer, A.; Priglinger, C.; Wolf, A.; Mayer, W.J.; Teupser, D.; Holdt, L.M.; Priglinger, S.G.; et al. Poly(lactic-co-glycolic) Acid as a Slow-Release Drug-Carrying Matrix for Methotrexate Coated onto Intraocular Lenses to Conquer Posterior Capsule Opacification. Curr. Eye Res. 2018, 43, 702–708. [Google Scholar] [CrossRef]
- Manju, S.; Kunnatheeri, S. Layer-by-Layer modification of poly (methyl methacrylate) intra ocular lens: Drug delivery applications. Pharm. Dev. Technol. 2010, 15, 379–385. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Vieira, A.P.; Colaço, R.; Saramago, B.; Gil, M.H.; Coimbra, P.; Alves, P.; Bozukova, D.; Correia, T.R.; Correia, I.J.; et al. Controlled release of moxifloxacin from intraocular lenses modified by Ar plasma-assisted grafting with AMPS or SBMA: An in vitro study. Colloids Surf. B Biointerfaces 2017, 156, 95–103. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Haj-Ahmad, R.; Arshad, M.S.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Electrically atomised formulations of timolol maleate for direct and on-demand ocular lens coatings. Eur. J. Pharm. Biopharm. 2017, 119, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Development and characterisation of electrospun timolol maleate-loaded polymeric contact lens coatings containing various permeation enhancers. Int. J. Pharm. 2017, 532, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Singh, N.; van der Merwe, S.M.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019, 108, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Pinto, L.F.V.; Bozukova, D.; Santos, L.F.; Serro, A.P.; Saramago, B. Chitosan/alginate based multilayers to control drug release from ophthalmic lens. Colloids Surf. B Biointerfaces 2016, 147, 81–89. [Google Scholar] [CrossRef]
- Vieira, A.P.; Pimenta, A.F.R.; Silva, D.; Gil, M.H.; Alves, P.; Coimbra, P.; Mata, J.L.G.C.; Bozukova, D.; Correia, T.R.; Correia, I.J.; et al. Surface modification of an intraocular lens material by plasma-assisted grafting with 2-hydroxyethyl methacrylate (HEMA), for controlled release of moxifloxacin. Eur. J. Pharm. Biopharm. 2017, 120, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, Y.; Ma, H.; Zhang, C.; Fu, S. Comparison of posterior capsule opacification in rabbit eyes receiving different administrations of rapamycin. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1111–1118. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Q.; Li, J.; Han, Y.; Chang, P.; Lu, F.; Zhao, Y.E. ROCK inhibitor modified intraocular lens as an approach for inhibiting the proliferation and migration of lens epithelial cells and posterior capsule opacification. Biomater. Sci. 2019, 7, 4208–4217. [Google Scholar] [CrossRef]
- Han, Y.; Tang, J.; Xia, J.; Wang, R.; Qin, C.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Anti-adhesive and antiproliferative synergistic surface modification of intraocular lens for reduced posterior capsular opacification. Int. J. Nanomed. 2019, 14, 9047–9061. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Sousa, H.C.D.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Serro, A.P.; Saramago, B. Antibacterial layer-by-layer coatings to control drug release from soft contact lenses material. Int. J. Pharm. 2018, 553, 186–200. [Google Scholar] [CrossRef]
- Paradiso, P.; Colaço, R.; Mata, J.L.G.; Krastev, R.; Saramago, B.; Serro, A.P. Drug release from liposome coated hydrogels for soft contact lenses: The blinking and temperature effect. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 105, 1799–1807. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, W.; Lei, M.; He, Y.; Yan, M.; Zhang, X.; Zhao, C. Laser-triggered intraocular implant to induce photodynamic therapy for posterior capsule opacification prevention. Int. J. Pharm. 2016, 498, 1–11. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted materials as advanced excipients for drug delivery systems. Biotechnol. Annu. Rev. 2006, 12, 225–268. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Concheiro, A. Ocular drug delivery from molecularly-imprinted contact lenses. J. Drug Deliv. Sci. Technol. 2010, 20, 237–248. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended wear therapeutic contact lens fabricated from timolol imprinted carboxymethyl chitosan-g-hydroxy ethyl methacrylate-g-poly acrylamide as a onetime medication for glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, Y.; Han, S.; Zhao, Q.; Liu, N. Bimatoprost Imprinted Silicone Contact Lens to Treat Glaucoma. AAPS Pharmscitech 2020, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Bioinspired Imprinted PHEMA-Hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules 2011, 12, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Receptor-based biomimetic NVP/DMA contact lenses for loading/eluting carbonic anhydrase inhibitors. J. Membr. Sci. 2011, 383, 60–69. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Chen, J.; Ding, H.; Deng, D.; Xie, Z. Self-Reporting Colorimetric Analysis of Drug Release by Molecular Imprinted Structural Color Contact Lens. ACS Appl. Mater. Interfaces 2018, 10, 34611–34617. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Serro, A.P.; Silva, D.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrogels for diabetic eyes: Naltrexone loading, release profiles and cornea penetration. Mater. Sci. Eng. C 2019, 105, 10092. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef]
- White, C.J.; Tieppo, A.; Byrne, M.E. Controlled drug release from contact lenses: A comprehensive review from 1965-present. J. Drug Deliv. Sci. Technol. 2011, 21, 369–384. [Google Scholar] [CrossRef]
- Zaidi, S.A. Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 2016, 6, 88807–88819. [Google Scholar] [CrossRef]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling drug release from imprinted hydrogels by modifying the characteristics of the imprinted cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef]
- Bouledjouidja, A.; Masmoudi, Y.; Sergent, M.; Badens, E. Effect of operational conditions on the supercritical carbon dioxide impregnation of anti-inflammatory and antibiotic drugs in rigid commercial intraocular lenses. J. Supercrit. Fluids 2017, 130, 63–75. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Guerra, J.P.; Duarte, A.R.C.; Duarte, C.M.M.; Leite, E.O.B.; Gil, M.H.; de Sousa, H.C. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Duarte, C.M.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; de Sousa, H.C. Anti-glaucoma drug-loaded contact lenses prepared using supercritical solvent impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Bouledjouidja, A.; Masmoudi, Y.; Li, Y.; He, W.; Badens, E. Supercritical impregnation and optical characterization of loaded foldable intraocular lenses using supercritical fluids. J. Cataract Refract. Surg. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Tassaing, T.; Le-Bourdon, G.; Badens, E. Supercritical loading of gatifloxacin into hydrophobic foldable intraocular lenses—Process control and optimization by following in situ CO2 sorption and polymer swelling. Int. J. Pharm. 2020, 581, 119247. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef]
- Masmoudi, Y.; Ben Azzouk, L.; Forzano, O.; Andre, J.M.; Badens, E. Supercritical impregnation of intraocular lenses. J. Supercrit. Fluids 2011, 60, 98–105. [Google Scholar] [CrossRef]
- Yokozaki, Y.; Sakebe, J.; Ng, B.; Shimoyama, Y. Effect of temperature, pressure and depressurization rate on release profile of salicylic acid from contact lenses prepared by supercritical carbon dioxide impregnation. Chem. Eng. Res. Des. 2015, 100, 89–94. [Google Scholar] [CrossRef]
- Yañez, F.; Martikainen, L.; Braga, M.E.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Duarte, C.M.M.; Gil, M.H.; De Sousa, H.C. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater. 2011, 7, 1019–1030. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Chauhan, A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Zhang, K.; Moore, L.; Ho, D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano 2014, 8, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.J.; Kim, D.H.; Chang, W.S.; Vales, T.P.; Kim, J.W.; Kim, K.H.; Kim, J.K. Thermo-sensitive nanogel-laden bicontinuous microemulsion drug-eluting contact lenses. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, X.; Wang, Y.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Drug Eluting Intraocular Lens Surface Modification for PCO Prevention. Colloids Interface Sci. Commun. 2018, 24, 40–44. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Cai, J.P.; Ma, X.Y.; Li, Y.; Cheng, J.W.; Wei, R.L. Sustained release of 5-fluorouracil from chitosan nanoparticles surface modified intra ocular lens to prevent posterior capsule opacification: An in vitro and in vivo study. J. Ocul. Pharmacol Ther. 2013, 29, 208–215. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Patil, R.J.; Desai, A.R.; Shukla, M.R.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation. Acta Biomater. 2019, 86, 350–362. [Google Scholar] [CrossRef]
- Sharma, M.; Bhowmick, R.; Gappa-Fahlenkamp, H. Drug-Loaded Nanoparticles Embedded in a Biomembrane Provide a Dual-Release Mechanism for Drug Delivery to the Eye. J. Ocul. Pharmacol. Ther. 2016, 32, 565–573. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic drug delivery systems for antibiotherapy- A review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Mun, J.; Mok, J.W.; Jeong, S.; Cho, S.; Joo, C.K.; Hahn, S.K. Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, A.R.; Choksi, H.H.; Patil, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses. Int. J. Pharm. 2017, 524, 193–204. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, W.; Li, S.; Tao, X.; Mu, G. Bimatoprost loaded microemulsion laden contact lens to treat glaucoma. J. Drug Deliv. Sci. Technol. 2019, 54, 101330. [Google Scholar] [CrossRef]
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol loaded microemulsion laden silicone contact lens to manage glaucoma: in vitro and in vivo studies. J. Dispers. Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Ribeiro Filho, E.; Fialho, S.L.; Lucena, L.R.; Maia Filho, A.; Haddad, A.; Jorge, R.; Scott, I.U.; Da Silva Cunha, A. Pharmacokinetic and toxicity investigations of a new intraocular lens with a dexamethasone drug delivery system: A pilot study. Ophthalmologica 2006, 220, 338–342. [Google Scholar] [CrossRef]
- Tan, D.W.N.; Lim, S.G.; Wong, T.T.; Venkatraman, S.S. Sustained antibiotic-eluting intra-ocular lenses: A new approach. PLoS ONE 2016, 11, e0163857. [Google Scholar] [CrossRef] [Green Version]
- Eperon, S.; Rodriguez-Aller, M.; Balaskas, K.; Gurny, R.; Guex-Crosier, Y. A new drug delivery system inhibits uveitis in an animal model after cataract surgery. Int. J. Pharm. 2013, 443, 254–261. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Pandya, M.M.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Co-delivery of timolol and hyaluronic acid from semi-circular ring-implanted contact lenses for the treatment of glaucoma: in vitro and in vivo evaluation. Biomater. Sci. 2018, 6, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release. Mater. Sci. Eng. C 2020, 112, 110885. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. in vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Ciolino, J.B.; Ross, A.E.; Tulsan, R.; Watts, A.C.; Wang, R.F.; Zurakowski, D.; Serle, J.B.; Kohane, D.S. Latanoprost-Eluting Contact Lenses in Glaucomatous Monkeys. Ophthalmology 2016, 123, 2085–2092. [Google Scholar] [CrossRef]
- Song, C.; Ben-Shlomo, G.; Que, L. A Multifunctional Smart Soft Contact Lens Device Enabled by Nanopore Thin Film for Glaucoma Diagnostics and in Situ Drug Delivery. J. Microelectromech. Syst. 2019, 28, 810–816. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.H.Y.; Bui, B.V.; Vingrys, A.J. Clinical and experimental links between diabetes and glaucoma. Clin. Exp. Optom. 2011, 94, 4–23. [Google Scholar] [CrossRef]
- Hanyuda, A.; Sawada, N.; Yuki, K.; Uchino, M.; Ozawa, Y.; Sasaki, M.; Yamagishi, K.; Iso, H.; Tsubota, K.; Tsugane, S. Relationships of diabetes and hyperglycaemia with intraocular pressure in a Japanese population: The JPHC-NEXT Eye Study. Sci. Rep. 2020, 10, 5355. [Google Scholar] [CrossRef]
- Gerber, A.L.; Harris, A.; Siesky, B.; Lee, E.; Schaab, T.J.; Huck, A.; Amireskandari, A. Vascular Dysfunction in Diabetes and Glaucoma: A Complex Relationship Reviewed. J. Glaucoma 2015, 24, 474–479. [Google Scholar] [CrossRef]
- Capitena Young, C.E.; Kahook, M.Y.; Seibold, L.K. Novel Drug Delivery Systems for the Treatment of Glaucoma. Curr. Ophthalmol. Rep. 2019, 7, 143–149. [Google Scholar] [CrossRef]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Szigiato, A.A.; Podbielski, D.W.; Ahmed, I.I.K. Sustained drug delivery for the management of glaucoma. Expert Rev. Ophthalmol. 2017, 12, 173–186. [Google Scholar] [CrossRef]
- Sihota, R.; Angmo, D.; Ramaswamy, D.; Dada, T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J. Ophthalmol. 2018, 66, 495–505. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended Release of Timolol from Ethyl Cellulose Microparticles Laden Hydrogel Contact Lenses. Open Pharm. Sci. J. 2015, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mu, C.; Shi, M.; Liu, P.; Chen, L.; Marriott, G. Daylight-Mediated, Passive, and Sustained Release of the Glaucoma Drug Timolol from a Contact Lens. ACS Cent. Sci. 2018, 4, 1677–1687. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, K.S.; Kim, J.W.; Kang, J.Y.; Kim, J.K. Stimulus-responsive contact lens for IOP measurement or temperature-triggered drug release. Transl Vis. Sci. Technol. 2020, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Cheng, H.; Huo, Y.; Mao, S. Sustained ophthalmic delivery of highly soluble drug using pH-triggered inner layer-embedded contact lens. Int. J. Pharm. 2018, 544, 100–111. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Chauhan, A. Transport of topical anesthetics in vitamin e loaded silicone hydrogel contact lenses. Langmuir 2012, 28, 1478–1487. [Google Scholar] [CrossRef]
- Gupta, V.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of cataract: An appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef]
- McCarty, C.A. Cataract in the 21st century: Lessons from previous epidemiologic research. Clin. Exp. Optom. 2002, 85, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Laursen, S.B.; Erichsen, J.H.; Holm, L.M.; Kessel, L. Prevention of macular edema in patients with diabetes after cataract surgery. J. Cataract Refract. Surg. 2019, 45, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Schmidt-Erfurth, U. Diabetic Cataract—Pathogenesis, Epidemiology and Treatment. J. Ophthalmol. 2010, 2010, 608751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Cytosine-functionalized bioinspired hydrogels for ocular delivery of antioxidant transferulic acid. Biomater. Sci. 2020, 8, 1171–1180. [Google Scholar] [CrossRef]
- Dua, H.S.; Attre, R. Treatment of Post-operative Inflammation following Cataract Surgery—A Review. Eur. Ophthalmic Rev. 2012, 6, 98. [Google Scholar] [CrossRef]
- McCafferty, S.; Harris, A.; Kew, C.; Kassm, T.; Lane, L.; Levine, J.; Raven, M. Pseudophakic Pseudophakic cystoid macular edema prevention and risk factors; prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol. 2017, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, U.; Alm, A.; Bjärnhall, G.; Granstam, E.; Matsson, A.W. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 349–359. [Google Scholar] [CrossRef]
- Wielders, L.H.P.; Lambermont, V.A.; Schouten, J.S.A.G.; Van Den Biggelaar, F.J.H.M.; Worthy, G.; Simons, R.W.P.; Winkens, B.; Nuijts, R.M.M.A. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: A systematic review and meta-analysis. Am. J. Ophthalmol. 2015, 160, 968–981. [Google Scholar] [CrossRef]
- Khambhiphant, B.; Liumsirijarern, C.; Saehout, P. The effect of Nd: YAG laser treatment of posterior capsule opacification on anterior chamber depth and refraction in pseudophakic eyes. Clin. Ophthalmol. 2015, 9, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Praveen, M.R.; Vasavada, A.R.; Shah, G.D.; Shah, A.R.; Khamar, B.M.; Dave, K.H. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: A case-control study. Eye 2014, 28, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Donnenfeld, E.; Holland, E. Dexamethasone Intracameral Drug-Delivery Suspension for Inflammation Associated with Cataract Surgery: A Randomized, Placebo-Controlled, Phase III Trial. Ophthalmology 2018, 125, 799–806. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Serro, A.P.; Colaço, R.; Chauhan, A. Drug delivery to the eye anterior chamber by intraocular lenses: An in vivo concentration estimation model. Eur. J. Pharm. Biopharm. 2018, 133, 63–69. [Google Scholar] [CrossRef]
- Morarescu, D.; West-Mays, J.A.; Sheardown, H.D. Effect of delivery of MMP inhibitors from PDMS as a model IOL material on PCO markers. Biomaterials 2010, 31, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Fang, F.; Wu, B.; Zeng, L.; Cheng, Y. State of the art of intraocular lens manufacturing. Int. J. Adv. Manuf. Technol. 2018, 98, 1103–1130. [Google Scholar] [CrossRef]
- Tan, X.; Zhan, J.; Zhu, Y.; Cao, J.; Wang, L.; Liu, S.; Wang, Y.; Liu, Z.; Qin, Y.; Wu, M.; et al. Improvement of Uveal and Capsular Biocompatibility of Hydrophobic Acrylic Intraocular Lens by Surface Grafting with 2-Methacryloyloxyethyl Phosphorylcholine-Methacrylic Acid Copolymer. Sci. Rep. 2017, 7, 40462. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.S.; Bertrand, V.; Bozukova, D.; Pagnoulle, C.; Labrugère, C.; De Pauw, E.; De Pauw-Gillet, M.C.; Durrieu, M.C. RGD surface functionalization of the hydrophilic acrylic intraocular lens material to control posterior capsular opacification. PLoS ONE 2014, 9, e114973. [Google Scholar] [CrossRef] [Green Version]
- Tetz, M.; Jorgensen, M.R. New Hydrophobic IOL Materials and Understanding the Science of Glistenings. Curr. Eye Res. 2015, 40, 969–981. [Google Scholar] [CrossRef]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior eye segment drug delivery systems: Current treatments and future challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vision Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Clayton, J.A. Dry eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Findlay, Q.; Reid, K. Dry eye disease: When to treat and when to refer. Aust. Prescr. 2018, 41, 160–163. [Google Scholar] [CrossRef]
- Karsten, E. Diversity of Microbial Species Implicated in Keratitis: A Review. Open Ophthalmol. J. 2012, 6, 110–124. [Google Scholar] [CrossRef]
- Kuruvilla, S.; Peter, J.; David, S.; Premkumar, P.S.; Ramakrishna, K.; Thomas, L.; Vedakumar, M.; Peter, J.V. Incidence and risk factor evaluation of exposure keratopathy in critically ill patients: A cohort study. J. Crit. Care 2015, 30, 400–404. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Panfil, C.; Schrage, N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea 2015, 34, 560–566. [Google Scholar] [CrossRef]
- Daphna, O.; Mimouni, M.; Keshet, Y.; Ben Ishai, M.; Barequet, I.S.; Knyazer, B.; Mrukwa-Kominek, E.; Zarnowski, T.; Chen-Zion, M.; Marcovich, A. Therapeutic HL-Contact Lens versus Standard Bandage Contact Lens for Corneal Edema: A Prospective, Multicenter, Randomized, Crossover Study. J. Ophthalmol. 2020, 2020, 8410920. [Google Scholar] [CrossRef]
- Siu, G.D.J.Y.; Young, A.L.; Jhanji, V. Alternatives to corneal transplantation for the management of bullous keratopathy. Curr. Opin. Ophthalmol. 2014, 25, 347–352. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Kocaba, V.; Weiss, J.S.; Jurkunas, U.V.; Kinoshita, S.; Aldave, A.J.; Mehta, J.S. Corneal dystrophies. Nat. Rev. Dis. Prim. 2020, 6, 46. [Google Scholar] [CrossRef]
- Han, S.B.; Yang, H.K.; Hyon, J.Y. Influence of diabetes mellitus on anterior segment of the eye. Clin. Interv. Aging 2019, 14, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; Patel, D.V.; Mcghee, C.N.; Alany, R.G. New therapeutic approaches in the treatment of diabetic keratopathy: A review. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef]
- de Alves, M.C.; Carvalheira, J.B.; Módulo, C.M.; Rocha, E.M. Tear film and ocular surface changes in diabetes mellitus. Arq. Bras. Oftalmol. 2008, 71, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zidan, G.; Rupenthal, I.D.; Greene, C.; Seyfoddin, A. Medicated ocular bandages and corneal health: Potential excipients and active pharmaceutical ingredients. Pharm. Dev. Technol. 2018, 23, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Kilfoyle, D.; Braatvedt, G.D.; Craig, J.P. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J. Diabetes Res. 2014, 2014, 848659. [Google Scholar] [CrossRef] [Green Version]
- Shih, K.C.; Lam, K.L.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr. Diabetes 2017, 7, e251. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Byrne, M.E. Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses. Pharm. Res. 2009, 26, 714–726. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shaikh, A.A.; Lakdawala, D.H.; Desai, A.R.; Pandya, M.M.; Singhania, S.S.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: in vitro and in vivo evaluation. Acta Biomater. 2017, 53, 211–221. [Google Scholar] [CrossRef]
- White, C.J.; Dipasquale, S.A.; Byrne, M.E. Controlled Release of Multiple Therapeutics from Silicone Hydrogel Contact Lenses. Optom. Vis. Sci. 2016, 93, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, H.J.; Noh, H. pH sensitive soft contact lens for selective drug-delivery. Macromol. Res. 2018, 26, 278–283. [Google Scholar] [CrossRef]
- Pitt, W.G.; Jack, D.R.; Zhao, Y.; Nelson, J.L.; Pruitt, J.D. Loading and release of a phospholipid from contact lenses. Optom. Vis. Sci. 2011, 88, 502–506. [Google Scholar] [CrossRef]
- Pitt, W.G.; Jack, D.R.; Zhao, Y.; Nelson, J.L.; Pruitt, J.D. Transport of phospholipid in silicone hydrogel contact lenses. J. Biomater. Sci. Polym. Ed. 2012, 23, 527–541. [Google Scholar] [CrossRef]
- Pitt, W.G.; Zhao, Y.; Jack, D.R.; Perez, K.X.; Jones, P.W.; Marelli, R.; Nelson, J.L.; Pruitt, J.D. Extended elution of phospholipid from silicone hydrogel contact lenses. J. Biomater. Sci. Polym. Ed. 2015, 26, 224–234. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Li, J.; Wang, Y.; Chen, Q.; Hou, C.; Al, E. Efficacy of osmoprotectants on prevention and treatment of murine dry eye. Investig. Ophth Vis. Sci. 2013, 54, 6287–6297. [Google Scholar] [CrossRef] [Green Version]
- Hsu, K.H.; De La Jara, P.L.; Ariyavidana, A.; Watling, J.; Holden, B.; Garrett, Q.; Chauhan, A. Release of betaine and dexpanthenol from vitamin E modified silicone-hydrogel contact lenses. Curr. Eye Res. 2015, 40, 267–273. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Deng, S.; Sun, X.; Wang, N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J. Ophthalmol. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Aranguez, A.; Fonseca, B.; Carracedo, G.; Martin-Gil, A.; Martinez-Aguila, A.; Pintor, J. Dry Eye Treatment Based on Contact Lens Drug Delivery: A Review. Eye Contact Lens 2016, 42, 280–288. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.; Jones, L. Contact lenses for antifungal ocular drug delivery: A review. Expert Opin. Drug Deliv. 2014, 11, 537–546. [Google Scholar] [CrossRef]
- Garg, P.; Venuganti, V.V.K.; Roy, A.; Roy, G. Novel drug delivery methods for the treatment of keratitis: Moving away from surgical intervention. Expert Opin. Drug Deliv. 2019, 16, 1381–1391. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Qu, C.; Bo, G.; Jiang, L.; Zhao, H.; Zhang, J.; Lin, Y.; Hua, Y.; Yang, P.; et al. A Mussel-Inspired Facile Method to Prepare Multilayer-AgNP-Loaded Contact Lens for Early Treatment of Bacterial and Fungal Keratitis. ACS Biomater. Sci. Eng. 2018, 4, 1568–1579. [Google Scholar] [CrossRef]
- Aveyard, J.; Deller, R.C.; Lace, R.; Williams, R.L.; Kaye, S.B.; Kolegraff, K.N.; Curran, J.M.; D’Sa, R.A. Antimicrobial Nitric Oxide Releasing Contact Lens Gels for the Treatment of Microbial Keratitis. ACS Appl. Mater. Interfaces 2019, 11, 37491–37501. [Google Scholar] [CrossRef]
- Hewitt, M.G.; Morrison, P.W.J.; Boostrom, H.M.; Morgan, S.R.; Fallon, M.; Lewis, P.N.; Whitaker, D.; Brancale, A.; Varricchio, C.; Quantock, A.J.; et al. in vitro Topical Delivery of Chlorhexidine to the Cornea: Enhancement Using Drug-Loaded Contact Lenses and β-Cyclodextrin Complexation, and the Importance of Simulating Tear Irrigation. Mol. Pharm. 2020, 17, 1428–1441. [Google Scholar] [CrossRef]
- Huang, J.F.; Zhong, J.; Chen, G.P.; Lin, Z.T.; Deng, Y.; Liu, Y.L.; Cao, P.Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef]
- Gallagher, A.G.; McLean, K.; Stewart, R.M.K.; Wellings, D.A.; Allison, H.E.; Williams, R.L. Development of a poly-ε-lysine contact lens as a drug delivery device for the treatment of fungal keratitis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4499–4505. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of fluconazole from contact lenses using a novel in vitro eye model. Optom Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef]
- Jaishankar, D.; Buhrman, J.S.; Valyi-Nagy, T.; Gemeinhart, R.A.; Shukla, D. Extended release of an anti–heparan sulfate peptide from a contact lens suppresses corneal herpes simplex virus-1 infection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted contact lenses for ocular administration of antiviral drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef]
- Jacob, S.; Kumar, D.A.; Agarwal, A.; Basu, S.; Sinha, P.; Agarwal, A. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J. Refract. Surg. 2014, 30, 366–372. [Google Scholar] [CrossRef]
- Kubrak-Kisza, M.; Kisza, K.J.; Misiuk-Hojło, M. Corneal Cross-Linking: An Example of Photoinduced Polymerization as a Treatment Modality in Keratoconus. Polim. Med. 2016, 46, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, C.; Jain, A.K.; Gupta, A.; Ram, J.; Ramatchandirane, B.; Dhingra, D.; Sachdeva, K.; Kumar, A. Demarcation line depth after contact lens–assisted corneal crosslinking for progressive keratoconus: Comparison of dextran-based and hydroxypropyl methylcellulose–based riboflavin solutions. J. Cataract Refract. Surg. 2017, 43, 1263–1270. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.-C.; Mohamed-Noriega, K.; Mehta, J.S. Application of Novel Drugs for Corneal Cell Regeneration. J. Ophthalmol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wollensak, G.; Spörl, E.; Herbst, H. Biomechanical efficacy of contact lens-assisted collagen cross-linking in porcine eyes. Acta Ophthalmol. 2019, 97, e84–e90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, S.; Morck, D.; Schultz, C. Treatment of corneal defects with delayed re-epithelization with a medical device/drug delivery system for epidermal growth factor. Clin. Exp. Ophthalmol. 2012, 40, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Delfino, A.; Riva, F.; Icaro Cornaglia, A.; Marrubini, G.; Musitelli, G.; Del Fante, C.; Perotti, C.; et al. Platelet lysate and chondroitin sulfate loaded contact lenses to heal corneal lesions. Int. J. Pharm. 2016, 509, 188–196. [Google Scholar] [CrossRef]
- Nakahara, M.; Miyata, K.; Otani, S.; Miyai, T.; Nejima, R.; Yamagami, S.; Amano, S. A randomised, placebo controlled clinical trial of the aldose reductase inhibitor CT-112 as management of corneal epithelial disorders in diabetic patients. Br. J. Ophthalmol. 2005, 89, 266–268. [Google Scholar] [CrossRef] [Green Version]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubotav, K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [Green Version]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative stress in diseases of the human cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Mandal, A.; Pal, D.; Mitra, A.K. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert Opin. Drug Deliv. 2017, 14, 1145–1162. [Google Scholar] [CrossRef]
- Rastogi, N.; Smith, R.T. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv Ophthalmol. 2016, 61, 422–433. [Google Scholar] [CrossRef]
- Mehta, S. Age-Related Macular Degeneration. Prim. Care-Clin. Off. Pract. 2015, 42, 377–391. [Google Scholar] [CrossRef]
- Dedania, V.S.; Grob, S.; Zhang, K.; Bakri, S.J. Pharmacogenomics of response to anti-vegf therapy in exudative age-related macular degeneration. Retina 2015, 35, 381–391. [Google Scholar] [CrossRef]
- Davidson, J.A.; Ciulla, T.A.; McGill, J.B.; Kles, K.A.; Anderson, P.W. How the diabetic eye loses vision. Endocrine 2007, 32, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Christopher, K.; Chauhan, A. Contact Lens Based Drug Delivery to the Posterior Segment Via Iontophoresis in Cadaver Rabbit Eyes. Pharm. Res. 2019, 36, 87. [Google Scholar] [CrossRef]
- Molokhia, S.A.; Sant, H.; Simonis, J.; Bishop, C.J.; Burr, R.M.; Gale, B.K.; Ambati, B.K. The capsule drug device: Novel approach for drug delivery to the eye. Vis. Res. 2010, 50, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yifan Yu, P.; Sekar, P.; Chauhan, A. Commercialization challenges for drug eluting contact lenses. Expert Opin. Drug Deliv. 2020, 17, 1133–1149. [Google Scholar] [CrossRef]


| Pharmacological Action | Drugs | Lens Type | Backbone Monomers | Ref. |
|---|---|---|---|---|
| Beta-adrenergic antagonist | Timolol | CL | HEMA | [82,102,113] |
| HEMA-MAA | [86,93,103,121,135] | |||
| HEMA-PVP | [54,93,136] | |||
| HEMA-PC | [93] | |||
| HEMA-DMA/GMA/Sil | [104,137] | |||
| Sil-DMA | [55] | |||
| Sil-DMA-MAA-PVP | [101] | |||
| Sil-DMA-HEMA | [116] | |||
| Sil-DMA-HEMA-PVP | [56,107,122,123] | |||
| Sil-DMA-HEMA-PVP-PDMS | [52,101] | |||
| Sil-PVP | [67,68,69,94] | |||
| PDMS | [126] | |||
| Modified PVA | [93] | |||
| Betaxolol | CL | Sil-HEMA-PVP | [138] | |
| Puerarin | CL | HEMA-PVP-MA | [61] | |
| Sympathomimetic agents | Brimonidine | CL | HEMA-PVP | [54] |
| Carbonic anhydrase inhibitors | Dorzolamide | CL | Sil-DMA-HEMA-PVP-PDMS | [52] |
| Ethoxzolamide | CL | HEMA | [51,84] | |
| PVP-DMA | [85] | |||
| Acetazolamide | CL | HEMA | [84] | |
| PVP-DMA | [85] | |||
| HEMA-PC/MAA/PVP | [93] | |||
| Modified PVA | [93] | |||
| Sil-PVP | [94] | |||
| Prostaglandin analogues | Latanoprost | CL | HEMA | [38,113] |
| HEMA-MAA | [124,125] | |||
| Sil-DMA/PVP | [38] | |||
| Sil-DMA-HEMA-PVP(-PDMS) | [38,53] | |||
| Sil-IBM-PVP-HBM-MVA | [38] | |||
| Bimatoprost | CL | Sil-DMA-HEMA | [115] | |
| Sil-DMA-HEMA-MAA | [83] | |||
| Sil-DMA-HEMA-PVP(-PDMS) | [53,123] |
| Pharmacological Action | Drugs | Lens Type | Backbone Monomers | Ref. | |
|---|---|---|---|---|---|
| Cataract prevention | Aldose-reductase inhibitor | Epalrestat | CL | Sil-HEMA | [88] |
| Antioxidant | Acetylcysteine | CL | HEMA-PVP | [37] | |
| Sil-DMA-HEMA-PVP | |||||
| HEMA-MAA | |||||
| Transferulic acid | CL | HEMA-GMA-EGPEM | [144] | ||
| Prophylaxis after cataract surgery | Antibiotics | Norfloxacin | IOL | HEMA | [23] |
| HEMA-BEM | [60] | ||||
| Moxifloxacin | IOL | HEMA-MMA | [24,39,40,41,42,66,70,71,153] | ||
| Silicone hydrogel (N/A) | [153] | ||||
| N/A | [118] | ||||
| Ciprofloxacin | IOL | HEMA | [26] | ||
| HEMA-MMA | [95] | ||||
| PolyMMA | [92] | ||||
| Gatifloxacin | IOL | HEMA-MMA | [39] | ||
| BMA-MMA | [96] | ||||
| Ampicillin | IOL | PolyMMA | [65] | ||
| Levofloxacin | IOL | HEMA-MMA | [153] | ||
| Silicone hydrogel (N/A) | [153] | ||||
| NSAIDs | Diclofenac | IOL | HEMA-MMA | [24,40,42,70,153] | |
| Silicone hydrogel (N/A) | [153] | ||||
| Ketorolac | IOL | HEMA-MMA | [24,40,70,153] | ||
| Silicone hydrogel (N/A) | [153] | ||||
| Steroidal anti-inflammatory drugs | Dexamethasone | IOL | HEMA | [26,95] | |
| HEMA-MMA | [25,95] | ||||
| PolyMMA | [92] | ||||
| Triamcinolone acetonide | IOL | PEA-PEMA | [119] | ||
| Immunosuppressant | Cyclosporine A | IOL | PEA-PEMA | [119] | |
| PCO prevention | Anti-proliferation or apoptosis-inducing drugs | Celecoxib | IOL | Hydrophilic acrylic (N/A) | [32] |
| Erufosine | IOL | Hydrophilic acrylic (N/A) | [33] | ||
| Erlotinib | IOL | Hydrophilic acrylic (N/A) | [34] | ||
| Hydrophobic acrylic (N/A) | |||||
| Gefitinib | IOL | Hydrophilic acrylic (N/A) | [35] | ||
| Hydrophobic acrylic (N/A) | |||||
| Methotrexate | IOL | Hydrophilic acrylic (N/A) | [36,64] | ||
| Hydrophobic acrylic (N/A) | [36,97] | ||||
| Rapamycin | IOL | PolyMMA | [72] | ||
| Y27632 | IOL | PEA-PEMA | [73] | ||
| Doxorubicin | IOL | Hydrophobic acrylic (N/A) | [74] | ||
| Hydrophobic polyester | [74,105] | ||||
| Indocyanine green | IOL | N/A | [77] | ||
| MMPI | IOL | PDMS | [154] | ||
| 5-fluorouracil | IOL | PolyMMA | [106] |
| Pharmacological Action | Drugs/Agents | Lens Type | Backbone Monomers | Ref. |
|---|---|---|---|---|
| Lubricating agents, moisturizing agents | HA | CL | HEMA-MAA | [176,177] |
| Modified PVA | [175] | |||
| HPMC | CL | HEMA(-PVP/pNIPAAm) | [179] | |
| Sil-DMA | [81] | |||
| Sil-DMA-PDMS | [178] | |||
| Phospholipids | CL | Silicone hydrogel (N/A) | [180,181,182] | |
| Dexpanthenol | CL | Sil-DMA-HEMA-PVP | [184] | |
| Sil-DMA-HEMA-PVP-PDMS | ||||
| Trehalose | CL | Sil-DMA-PDMS | [178] | |
| Osmoprotectant | Betaine | CL | Sil-DMA-HEMA-PVP | [184] |
| Sil-DMA-HEMA-PVP-PDMS | ||||
| Antibiotics and anti-inflammatory drugs | Corticosteroids, NSAIDs, cyclosporine A, antibiotics | CL | Various compositions | [21,178] |
| Keratitis medication | Biocides | CL | HEMA-MAA-PVP | [189] |
| Poly-ε-lysine | [190] | |||
| Antifungals | CL | Silicone hydrogel (N/A) | [191,194] | |
| Poly-ε-lysine | [193] | |||
| HEMA-PC | [194] | |||
| HEMA-MAA | [194] | |||
| HEMA-MAA-PVP | [189,194] | |||
| Sil-DMA-HEMA-PVP | [194] | |||
| Quaternized chitosan + graphene oxide | [192] | |||
| Antivirals | CL | HEMA-MAA | [195,196] | |
| Collagen photosensitizer | Riboflavin | CL | HEMA-PVP | [197,199,201] |
| Sil-DMA | [201] | |||
| PVP-MMA | [201] | |||
| Opioid antagonist | Naltrexone | CL | HEMA | [87] |
| Growth factor | EGF | CL | HEMA-PVP | [202] |
| Sil-DMA | [43] | |||
| PVP-MMA | [43] | |||
| PDGF | CL | Modified PVA | [203] | |
| Sil-PVP | ||||
| Sil-DMA | ||||
| Sil-DMA-HEMA-PVP-PDMS | ||||
| Aldose reductase inhibitor | Epalrestat | CL | Sil-HEMA | [88] |
| Antioxidant | Lactoferrin | CL | Silicone hydrogels (N/A) | [44,45] |
| Pharmacological Action | Drugs/Molecules | Lens Type | Backbone Monomers | Ref. |
|---|---|---|---|---|
| Steroidal anti-inflammatory drugs | Prednisolone, beclomethasone | CL | PVP-MMA | [31] |
| Dexamethasone | CL | HEMA-MAA | [120] | |
| Anesthetic | Lidocaine | CL | N/A | [108] |
| Anti-VEGF | Ranibizumab | CL | PVP-MMA | [31] |
| - | Nile blue, fluorescein (iontophoresis) | CL | Sil-DMA-HEMA-PVP-PDMS | [213] |
| Immunosuppressant | Cyclosporine A | IOL | PEA-PEMA | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics 2021, 13, 36. https://doi.org/10.3390/pharmaceutics13010036
Toffoletto N, Saramago B, Serro AP. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics. 2021; 13(1):36. https://doi.org/10.3390/pharmaceutics13010036
Chicago/Turabian StyleToffoletto, Nadia, Benilde Saramago, and Ana Paula Serro. 2021. "Therapeutic Ophthalmic Lenses: A Review" Pharmaceutics 13, no. 1: 36. https://doi.org/10.3390/pharmaceutics13010036
APA StyleToffoletto, N., Saramago, B., & Serro, A. P. (2021). Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics, 13(1), 36. https://doi.org/10.3390/pharmaceutics13010036