Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fusion Protein Antibody
2.2. Cell Lines
2.3. Conjugation
2.4. Mass Spectrometry Analysis
2.5. Radiolabeling
2.6. Purity and Stability
2.6.1. HPLC
2.6.2. iTLC
2.7. In Vitro Characterization of Immunoreactivity
2.8. In Vivo Characterization
2.8.1. Murine Xenograft Model
2.8.2. Biodistribution Studies
2.8.3. Pharmacokinetic Modeling
2.8.4. Murine Dosimetry
2.9. Statistics
3. Results
3.1. Conjugation and Radiolabeling
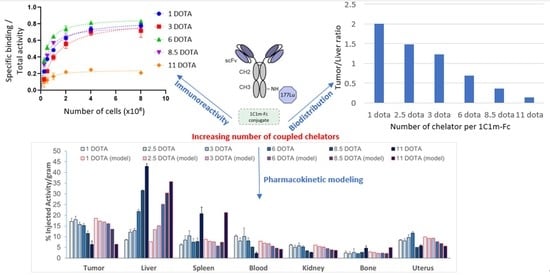
3.2. Immunoreactive Fraction
3.3. In Vivo Characterization
3.3.1. Biodistribution Study at 24 h
3.3.2. Complementary Analyses for 1C1m-(DOTA)1 and 1C1m-(DOTA)3
3.3.3. Pharmacokinetic Modeling
3.3.4. Murine Dosimetry
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Model Description



| 1 DOTA | 3 DOTA | Simultaneous Fit | 1 DOTA | 3 DOTA | Simultaneous Fit | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Value | ± | SD | Value | ± | SD | Value | ± | SD | Parameter | Value | ± | SD | Value | ± | SD | Value | ± | SD |
| K2:1 | 1.01 × 10−1 | ± | 7.31 × 10−3 | 2.67 × 10−1 | ± | 2.92 × 10−2 | 1.08 × 10−1 | ± | 1.65 × 10−2 | BTu | 3.68 × 10−2 | ± | 1.98 × 10−3 | 1.41 × 10−2 | ± | 1.88 × 10−3 | 4.74 × 10−3 | ± | 3.21 × 10−3 |
| K1:2 | 7.65 × 10−2 | ± | 4.28 × 10−3 | 2.76 × 10−1 | ± | 3.28 × 10−2 | 8.11 × 10−2 | ± | 1.01 × 10−2 | BLu | 2.75 × 10−2 | ± | 1.25 × 10−3 | 2.75 × 10−2 | ± | 1.54 × 10−3 | 2.38 × 10−2 | ± | 3.03 × 10−3 |
| K3:1 | 1.63 × 10−2 | ± | 7.06 × 10−4 | 2.37 × 10−2 | ± | 1.30 × 10−3 | 9.27 × 10−3 | ± | 2.87 × 10−3 | BLi | 2.49 × 10−1 | ± | 8.67 × 10−3 | 2.75 × 10−1 | ± | 1.46 × 10−2 | 1.83 × 10−1 | ± | 2.05 × 10−2 |
| K1:3 | 1.07 × 10−2 | ± | 6.96 × 10−4 | 2.31 × 10−2 | ± | 1.20 × 10−3 | 2.33 × 10−2 | ± | 2.44 × 10−3 | BSp | 1.65 × 10−2 | ± | 7.39 × 10−4 | 1.68 × 10−2 | ± | 9.29 × 10−4 | 1.51 × 10−2 | ± | 1.73 × 10−3 |
| K4:1 | 3.85 × 10−3 | ± | 4.44 × 10−4 | 3.32 × 10−3 | ± | 7.04 × 10−4 | 7.97 × 10−3 | ± | 1.87 × 10−3 | BKi | 4.85 × 10−2 | ± | 2.12 × 10−3 | 5.38 × 10−2 | ± | 2.78 × 10−3 | 4.93 × 10−2 | ± | 5.31 × 10−3 |
| K1:4 | 2.06 × 10−2 | ± | 2.24 × 10−3 | 3.03 × 10−2 | ± | 5.00 × 10−3 | 3.98 × 10−2 | ± | 8.80 × 10−3 | BHe | 1.43 × 10−2 | ± | 6.17 × 10−4 | 1.54 × 10−2 | ± | 8.02 × 10−4 | 1.36 × 10−2 | ± | 1.63 × 10−3 |
| K5:1 | 3.18 × 10−3 | ± | 1.02 × 10−4 | 9.90 × 10−3 | ± | 6.29 × 10−4 | 7.62 × 10−3 | ± | 5.80 × 10−4 | BMu | 2.07 × 10−3 | ± | 1.53 × 10−4 | 2.62 × 10−3 | ± | 1.96 × 10−4 | 2.23 × 10−3 | ± | 3.70 × 10−4 |
| K1:5 | 0.00 | ± | NA | 1.25 × 10−2 | ± | 9.88 × 10−4 | 1.76 × 10−2 | ± | 1.94 × 10−3 | BBo | 2.35 × 10−3 | ± | 1.16 × 10−4 | 2.99 × 10−3 | ± | 1.53 × 10−4 | 2.49 × 10−3 | ± | 2.90 × 10−4 |
| K6:1 | 4.20 × 10−3 | ± | 3.06 × 10−4 | 7.83 × 10−3 | ± | 6.85 × 10−4 | 6.36 × 10−2 | ± | 6.77 × 10−3 | BSt | 9.96 × 10−3 | ± | 5.69 × 10−4 | 8.55 × 10−3 | ± | 6.54 × 10−4 | 8.84 × 10−3 | ± | 1.34 × 10−3 |
| K1:6 | 8.50 × 10−3 | ± | 9.92 × 10−4 | 1.95 × 10−2 | ± | 1.70 × 10−3 | 1.77 × 10−2 | ± | 2.60 × 10−3 | BSi | 6.85 × 10−2 | ± | 3.37 × 10−3 | 7.03 × 10−2 | ± | 4.05 × 10−3 | 6.69 × 10−2 | ± | 8.29 × 10−3 |
| K7:1 | 3.68 × 10−3 | ± | 2.50 × 10−4 | 3.54 × 10−3 | ± | 3.78 × 10−4 | 4.91 × 10−3 | ± | 6.68 × 10−4 | BCo | 4.14 × 10−2 | ± | 2.00 × 10−3 | 4.14 × 10−2 | ± | 2.41 × 10−3 | 3.74 × 10−2 | ± | 4.94 × 10−3 |
| K1:7 | 4.72 × 10−3 | ± | 8.76 × 10−4 | 1.50 × 10−2 | ± | 1.66 × 10−3 | 1.32 × 10−2 | ± | 2.45 × 10−3 | BOv | 7.59 × 10−3 | ± | 2.54 × 10−4 | 2.48 × 10−3 | ± | 2.32 × 10−4 | 3.33 × 10−3 | ± | 5.24 × 10−4 |
| K8:1 | 1.03 × 10−3 | ± | 2.24 × 10−4 | 1.37 × 10−3 | ± | 3.52 × 10−4 | 3.28 × 10−3 | ± | 1.09 × 10−3 | BUt | 2.65 × 10−2 | ± | 1.14 × 10−3 | 1.08 × 10−2 | ± | 7.19 × 10−4 | 1.17 × 10−2 | ± | 1.57 × 10−3 |
| K1:8 | 1.58 × 10−2 | ± | 3.35 × 10−3 | 2.42 × 10−2 | ± | 4.96 × 10−3 | 3.68 × 10−2 | ± | 1.12 × 10−2 | BBl | 7.88 × 10−4 | ± | 5.44 × 10−5 | 5.88 × 10−4 | ± | 6.41 × 10−5 | 6.32 × 10−4 | ± | 1.15 × 10−4 |
| K9:1 | 1.53 × 10−3 | ± | 1.00 × 10−4 | 9.49 × 10−4 | ± | 1.01 × 10−4 | 1.46 × 10−3 | ± | 2.07 × 10−4 | Kel | 3.51 × 10−2 | ± | 5.48 × 10−4 | 4.23 × 10−2 | ± | 6.64 × 10−4 | 4.53 × 10−2 | ± | 1.54 × 10−3 |
| K1:9 | 1.07 × 10−2 | ± | 1.08 × 10−3 | 1.53 × 10−2 | ± | 1.85 × 10−3 | 1.49 × 10−2 | ± | 2.92 × 10−3 | ATIR | 2.57 × 10−2 | ± | 3.74 × 10−3 | ||||||
| K10:1 | 1.51 × 10−3 | ± | 1.33 × 10−4 | 1.14 × 10−3 | ± | 1.32 × 10−4 | 1.09 × 10−2 | ± | 1.58 × 10−3 | ASIR | 6.37 × 10−2 | ± | 7.45 × 10−3 | ||||||
| K1:10 | 4.42 × 10−3 | ± | 1.09 × 10−3 | 6.08 × 10−3 | ± | 1.45 × 10−3 | 7.62 × 10−3 | ± | 2.48 × 10−3 | ABIR | 1.05 × 10−2 | ± | 1.75 × 10−3 | ||||||
| K11:1 | 1.26 × 10−3 | ± | 1.04 × 10−4 | 9.95 × 10−4 | ± | 1.02 × 10−4 | 1.78 × 10−3 | ± | 2.62 × 10−4 | AUIR | 1.68 × 10−3 | ± | 2.31 × 10−3 | ||||||
| K1:11 | 9.67 × 10−3 | ± | 1.26 × 10−3 | 1.46 × 10−2 | ± | 1.65 × 10−3 | 1.80 × 10−2 | ± | 3.28 × 10−3 | ||||||||||
| K12:1 | 9.21 × 10−4 | ± | 9.60 × 10−5 | 8.57 × 10−4 | ± | 1.10 × 10−4 | 1.49 × 10−3 | ± | 2.57 × 10−4 | ||||||||||
| K1:12 | 6.19 × 10−3 | ± | 1.36 × 10−3 | 9.86 × 10−3 | ± | 1.75 × 10−3 | 1.30 × 10−2 | ± | 3.08 × 10−3 | ||||||||||
| K13:1 | 7.66 × 10−4 | ± | 7.69 × 10−5 | 8.51 × 10−4 | ± | 1.06 × 10−4 | 1.72 × 10−3 | ± | 2.76 × 10−4 | ||||||||||
| K1:13 | 2.28 × 10−3 | ± | 1.21 × 10−3 | 1.02 × 10−2 | ± | 1.73 × 10−3 | 1.52 × 10−2 | ± | 3.24 × 10−3 | ||||||||||
| K14:1 | 1.49 × 10−3 | ± | 6.42 × 10−5 | 3.81 × 10−3 | ± | 2.60 × 10−4 | 4.80 × 10−3 | ± | 5.90 × 10−4 | ||||||||||
| K1:14 | 0.00 | ± | NA | 1.68 × 10−2 | ± | 1.25 × 10−3 | 1.57 × 10−2 | ± | 2.50 × 10−3 | ||||||||||
| K15:1 | 6.36 × 10−3 | ± | 3.76 × 10−4 | 9.85 × 10−3 | ± | 5.63 × 10−4 | 9.90 × 10−3 | ± | 1.87 × 10−3 | ||||||||||
| K1:15 | 2.98 × 10−3 | ± | 7.11 × 10−4 | 1.27 × 10−2 | ± | 9.37 × 10−4 | 1.01 × 10−2 | ± | 1.51 × 10−3 | ||||||||||
| K16:1 | 5.86 × 10−3 | ± | 2.74 × 10−4 | 6.82 × 10−3 | ± | 4.16 × 10−4 | 6.38 × 10−3 | ± | 5.53 × 10−4 | ||||||||||
| K1:16 | 8.19 × 10−3 | ± | 7.14 × 10−4 | 1.75 × 10−2 | ± | 1.20 × 10−3 | 1.10 × 10−2 | ± | 1.64 × 10−3 | ||||||||||
References
- Shin, I.S.; Lee, S.-M.; Kim, H.S.; Yao, Z.; Regino, C.; Sato, N.; Cheng, K.T.; Hassan, R.; Campo, M.F.; Albone, E.F.; et al. Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody. Nucl. Med. Biol. 2011, 38, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. (Russ) Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef] [PubMed]
- Wojdowska, W.; Karczmarczyk, U.; Balog, L.; Sawicka, A.; Pöstényi, Z.; Kovács-Haász, V.; Polyák, A.; Laszuk, E.; Mikołajczak, R.; Garnuszek, P. Impact of DOTA-Chelators on the Antitumor Activity of 177Lu-DOTA-Rituximab Preparations in Lymphoma Tumor-Bearing Mice. Cancer Biother. Radiopharm. 2020, 35, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Bhadwal, M.; Das, T.; Kumar, C.; Sharma, R.; Amirdhanayagam, J.; Sarma, H.D.; Dash, A. Effect of Number of Bifunctional Chelating Agents on the Pharmacokinetics and Immunoreactivity of 177Lu-labeled Rituximab: A Systemic Study. Anti Cancer Agents Med. Chem. 2018, 18, 146–153. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S. Bifunctional Chelators for Therapeutic Lanthanide Radiopharmaceuticals. Bioconjug. Chem. 2001, 12, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Leitao, C.D.; Gentry, J.; Mitran, B.; Abouzayed, A.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Increase in negative charge of 68Ga/chelator complex reduces unspecific hepatic uptake but does not improve imaging properties of HER3-targeting affibody molecules. Sci. Rep. 2019, 9, 17710. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A. Influence of labelling methods on biodistribution and imaging properties of radiolabelled peptides for visualisation of molecular therapeutic targets. Curr. Med. Chem. 2010, 17, 2636–2655. [Google Scholar]
- Knogler, K.; Grünberg, J.; Novak-Hofer, I.; Zimmermann, K.; Schubiger, P.A. Evaluation of 177Lu-DOTA-labeled aglycosylated monoclonal anti-L1-CAM antibody chCE7: Influence of the number of chelators on the in vitro and in vivo properties. Nucl. Med. Biol. 2006, 33, 883–889. [Google Scholar] [CrossRef]
- Al-Ejeh, F.; Darby, J.M.; Thierry, B.; Brown, M.P. A simplified suite of methods to evaluate chelator conjugation of antibodies: Effects on hydrodynamic radius and biodistribution. Nucl. Med. Biol. 2009, 36, 395–402. [Google Scholar] [CrossRef]
- Elashoff, M.R.; Wingrove, J.; Beineke, P.; Daniels, S.; Tingley, W.G.; Rosenberg, S.; Voros, S.; Kraus, W.E.; Ginsburg, G.S.; Schwartz, R.S.; et al. Development of a blood-based gene expression algorithm for assessment of obstructive coronary artery disease in non-diabetic patients. BMC Med. Genom. 2011, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Teicher, B.A. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget 2019, 10, 993–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S.; Ahorn, H.; Novatchkova, M.; Garin-Chesa, P.; Park, J.E.; Weber, G.; Eisenhaber, F.; Rettig, W.J.; Lenter, M.C. Molecular Cloning and Characterization of EndoGlyx-1, an EMILIN-like Multisubunit Glycoprotein of Vascular Endothelium. J. Biol. Chem. 2001, 276, 48588–48595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkowicz, B.; Rybinski, K.; Foley, B.; Ebel, W.; Kline, B.; Routhier, E.; Sass, P.; Nicolaides, N.C.; Grasso, L.; Zhou, Y. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc. Natl. Acad. Sci. USA 2007, 104, 17965–17970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, M.; Devriese, A.; Jan, T.; Moons, M.; Lories, R.J.; Tavernier, J.; Conway, E.M. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer 2011, 11, 162. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Fujihara, A.; Natori, K.; Abe, A.; Kuboki, Y.; Higuchi, Y.; Aizawa, M.; Kuwata, T.; Kinoshita, T.; Yasui, W.; et al. TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Med. 2015, 4, 1667–1678. [Google Scholar] [CrossRef]
- Davies, G.; Cunnick, G.H.; Mansel, R.E.; Mason, M.D.; Jiang, W.G. Levels of expression of endothelial markers specific to tumour-associated endothelial cells and their correlation with prognosis in patients with breast cancer. Clin. Exp. Metastasis 2004, 21, 31–37. [Google Scholar] [CrossRef]
- Simonavicius, N.; Robertson, D.; Bax, D.; Jones, C.; Huijbers, I.J.; Isacke, C.M. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod. Pathol. 2008, 21, 308–315. [Google Scholar] [CrossRef]
- Nanda, A.; Karim, B.; Peng, Z.; Liu, G.; Qiu, W.; Gan, C.; Vogelstein, B.; Croix, B.S.; Kinzler, K.W.; Huso, D.L. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 3351–3356. [Google Scholar] [CrossRef] [Green Version]
- Delage, J.A.; Faivre-Chauvet, A.; Fierle, J.K.; Gnesin, S.; Schaefer, N.; Coukos, G.; Dunn, S.M.; Viertl, D.; Prior, J.O. (177)Lu radiolabeling and preclinical theranostic study of 1C1m-Fc: An anti-TEM-1 scFv-Fc fusion protein in soft tissue sarcoma. EJNMMI Res. 2020, 10, 98. [Google Scholar]
- Fierle, J.K.; Abram-Saliba, J.; Brioschi, M.; Detiani, M.; Coukos, G.; Dunn, S.M. Integrating SpyCatcher/SpyTag covalent fusion technology into phage display workflows for rapid antibody discovery. Sci. Rep. 2019, 9, 12815. [Google Scholar] [CrossRef] [Green Version]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A., Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar]
- Watabe, H.; Channing, M.A.; Der, M.G.; Adams, H.R.; Jagoda, E.M.; Herscovitch, P.; Eckelman, W.C.; Carson, R.E. Kinetic Analysis of the 5-HT2A Ligand [11C]MDL 100,907. Br. J. Pharmacol. 2000, 20, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A.; Greif, P.; Boston, R.C. WinSAAM: Application and explanation of use. In Mathematical Modeling in Nutrition and the Health Sciences; Springer: Boston, MA, USA, 2003; pp. 343–351. [Google Scholar] [CrossRef]
- Chu, S.C.; Berman, M. An exponential method for the solution of systems of ordinary differential equations. Commun. ACM 1974, 17, 699–702. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Gano, L.; Melo, R.; Mendes, F.; Oliveira, M.C.; Denoël, T.; Schaefer, N.; Viertl, D.; Fierle, J.; Coukos, G.; et al. Biological evaluation of new TEM1 targeting recombinant antibodies for radioimmunotherapy: In vitro, in vivo and in silico studies. Eur. J. Pharm. Biopharm. 2021, 158, 233–244. [Google Scholar] [CrossRef]
- Chacko, A.-M.; Li, C.; Nayak, M.; Mikitsh, J.L.; Hu, J.; Hou, C.; Grasso, L.; Nicolaides, N.C.; Muzykantov, V.R.; Divgi, C.R.; et al. Development of 124I Immuno-PET Targeting Tumor Vascular TEM1/Endosialin. J. Nucl. Med. 2014, 55, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Garnett, M.C. Targeted drug conjugates: Principles and progress. Adv. Drug Deliv. Rev. 2001, 53, 171–216. [Google Scholar] [CrossRef]
- Grunberg, J.; Jeger, S.; Sarko, D.; Dennler, P.; Zimmermann, K.; Mier, W.; Schibli, R. DOTA-Functionalized Polylysine: A High Number of DOTA Chelates Positively Influences the Biodistribution of Enzymatic Conjugated Anti-Tumor Antibody chCE7agl. PLoS ONE 2013, 8, e60350. [Google Scholar] [CrossRef] [Green Version]
- Wangler, C.; Moldenhauer, G.; Eisenhut, M.; Haberkorn, U.; Mier, W. Antibody−Dendrimer Conjugates: The Number, Not the Size of the Dendrimers, Determines the Immunoreactivity. Bioconjug. Chem. 2008, 19, 813–820. [Google Scholar] [CrossRef]
- Fischer, E.; Grünberg, J.; Cohrs, S.; Hohn, A.; Waldner-Knogler, K.; Jeger, S.; Zimmermann, K.; Novak-Hofer, I.; Schibli, R. L1-CAM-targeted antibody therapy and 177Lu-radioimmunotherapy of disseminated ovarian cancer. Int. J. Cancer 2011, 130, 2715–2721. [Google Scholar] [CrossRef]
- Mindt, T.L.; Jungi, V.; Wyss, S.; Friedli, A.; Pla, G.; Novak-Hofer, I.; Grünberg, J.; Schibli, R. Modification of Different IgG1 Antibodies via Glutamine and Lysine using Bacterial and Human Tissue Transglutaminase. Bioconjug. Chem. 2008, 19, 271–278. [Google Scholar] [CrossRef]
- Grunberg, J.; Novak-Hofer, I.; Honer, M.; Zimmermann, K.; Knogler, K.; Blauenstein, P.; Ametamey, S.; Maecke, H.R.; Schubiger, P.A. In vivo evaluation of 177Lu- and 67/64Cu-labeled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM-positive tumors. Clin. Cancer Res. 2005, 11, 5112–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gog, F.B.; Visser, G.W.; Klok, R.; van der Schors, R.; Snow, G.B.; van Dongen, G.A. Monoclonal antibodies labeled with rhenium-186 using the MAG3 chelate: Relationship between the number of chelated groups and biodistribution characteristics. J. Nucl. Med. 1996, 37, 352–362. [Google Scholar] [PubMed]
- Dearling, J.L.; Paterson, B.M.; Akurathi, V.; Betanzos-Lara, S.; Treves, S.T.; Voss, S.D.; White, J.M.; Huston, J.S.; Smith, S.V.; Donnelly, P.S.; et al. The Ionic Charge of Copper-64 Complexes Conjugated to an Engineered Antibody Affects Biodistribution. Bioconjug. Chem. 2015, 26, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Dahlsson Leitao, C.; Rinne, S.S.; Mitran, B.; Vorobyeva, A.; Andersson, K.G.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Molecular Design of HER3-Targeting Affibody Molecules: Influence of Chelator and Presence of HEHEHE-Tag on Biodistribution of (68)Ga-Labeled Tracers. Int. J. Mol. Sci. 2019, 20, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranski, A.-C.; Schäfer, M.; Bauder-Wüst, U.; Wacker, A.; Schmidt, J.; Liolios, C.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Kopka, K.; et al. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjug. Chem. 2017, 28, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Denoel, T.; Gnesin, S.; Riggi, N.; Irving, M.; Jakka, G.; Schaefer, N.; Viertl, D.; Coukos, G.; Prior, J.O. Preclinical Evaluation and Dosimetry of [(111)In]CHX-DTPA-scFv78-Fc Targeting Endosialin/Tumor Endothelial Marker 1 (TEM1). Mol. Imaging Biol. 2020, 22, 979–991. [Google Scholar] [CrossRef] [Green Version]



| Compound | Mass Weight (Da) | Estimated Number of DOTA per 1C1m-Fc | % Purity (HPLC) |
|---|---|---|---|
| Unmodified 1C1m-Fc | 108,394 | NA (not applicable) | 97.4% |
| DOTA (- HCl-H2O) | 551 | NA | NA |
| 1C1m-Fc 5 eq DOTA | 108,395–108,985 | 1 | 95.6% |
| 1C1m-Fc 10 eq DOTA | 108,986–110,758 | 2.5 | 96.2% |
| 1C1m-Fc 20 eq DOTA | 109,496–111,746 | 3 | 95.7% |
| 1C1m-Fc 30 eq DOTA | 110,755–113,117 | 6 | 96.9% |
| 1C1m-Fc 40 eq DOTA | 111,746–114,664 | 8.5 | 96.2% |
| 1C1m-Fc 50 eq DOTA | 113,711–116,068 | 11 | 96.8% |
| Number of DOTA per 1C1m-Fc | Immunoreactivity (%) ± SEM |
|---|---|
| 1 | 85.1 ± 1.3 |
| 3 | 86.2 ± 2.7 |
| 6 | 87.5 ± 1.0 |
| 8.5 | 78 ± 1.4 |
| 11 | 24 ± 1.7 |
| Organ | Mean Organ Mass (g) | TIAC (MBq·h/MBq) | Abs. Dose (mGy/MBq) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Brain t | 0.50 | - | - | 4.08 × 102 | 2.70 × 101 |
| Large intestine s,t | 0.78 | 1.98 | 0.33 | 7.03 × 102 | 8.10 × 101 |
| Small intestine s,t | 1.20 | 3.33 | 0.25 | 5.77 × 102 | 4.00 × 101 |
| Stomach s,t | 0.26 | 0.77 | 0.03 | 1.66 × 103 | 8.00 × 101 |
| Heart t | 0.11 | 0.48 | 0.06 | 1.10 × 103 | 1.50 × 102 |
| Heart content s | 0.2 | 1.84 | 0.35 | ||
| Kidneys s,t | 0.31 | 3.37 | 0.13 | 1.32 × 103 | 5.00 × 101 |
| Liver s,t | 1.13 | 21.49 | 1.72 | 1.79 × 103 | 1.30 × 102 |
| Lungs s,t | 0.15 | 1.03 | 0.31 | 9.83 × 102 | 2.07 × 102 |
| Pancreas t | 0.30 | 4.41 × 102 | 2.80 × 101 | ||
| Skeleton t | 2.20 | 4.18 × 102 | 2.80 × 101 | ||
| Spleen s,t | 0.10 | 1.06 | 0.03 | 1.18 × 103 | 1.00 × 102 |
| Ovaries s,* | 0.04 | 0.41 | 0.08 | 7.42 × 102 | 9.90 × 101 |
| Uterus s,* | 0.11 | 2.54 | 0.24 | 1.83 × 103 | 1.40 × 102 |
| Testes t | 0.16 | 4.09 × 102 | 2.60 × 101 | ||
| Thyroid t | 0.01 | 4.09 × 102 | 2.70 × 101 | ||
| Salivary glands s,* | 0.11 | 0.75 | 0.02 | 5.41 × 102 | 1.70 × 101 |
| Urinary Bladder s,t | 0.02 | 0.17 | 0.01 | 5.34 × 102 | 3.70 × 101 |
| Total Body s,t | 18.44 | 111.08 | 6.54 | 5.49 × 102 | 3.80 × 101 |
| Tumor s,* | 0.21 | 6.81 | 0.71 | 2.53 × 103 | 2.50 × 102 |
| Source Organ | Absorbed Dose (mGy/MBq) | |
|---|---|---|
| 1 DOTA | 3 DOTA | |
| Tumor SK-N-AS | 2.53 × 103 ± 2.50 × 102 | 1.82 × 103 ± 3.23 × 102 |
| Liver | 1.79 × 103 ± 1.30 × 102 | 2.23 × 103 ± 3.99 × 102 |
| Kidneys | 1.32 × 103 ± 5.00 × 101 | 7.05 × 102 ± 6.03 × 101 |
| Lungs | 9.83 × 102 ± 2.07 × 102 | 5.39 × 102 ± 1.30 × 102 |
| Spleen | 1.18 × 103 ± 1.00 × 102 | 1.20 × 103 ± 7.51 × 101 |
| Uterus | 1.83 × 103 ± 1.40 × 102 | 1.50 × 103 ± 5.15 × 102 |
| Tumor/Liver ratio | 1.4 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delage, J.A.; Faivre-Chauvet, A.; Barbet, J.; Fierle, J.K.; Schaefer, N.; Coukos, G.; Viertl, D.; Dunn, S.M.; Gnesin, S.; Prior, J.O. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics 2021, 13, 96. https://doi.org/10.3390/pharmaceutics13010096
Delage JA, Faivre-Chauvet A, Barbet J, Fierle JK, Schaefer N, Coukos G, Viertl D, Dunn SM, Gnesin S, Prior JO. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics. 2021; 13(1):96. https://doi.org/10.3390/pharmaceutics13010096
Chicago/Turabian StyleDelage, Judith Anna, Alain Faivre-Chauvet, Jacques Barbet, Julie Katrin Fierle, Niklaus Schaefer, George Coukos, David Viertl, Steven Mark Dunn, Silvano Gnesin, and John O. Prior. 2021. "Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model" Pharmaceutics 13, no. 1: 96. https://doi.org/10.3390/pharmaceutics13010096
APA StyleDelage, J. A., Faivre-Chauvet, A., Barbet, J., Fierle, J. K., Schaefer, N., Coukos, G., Viertl, D., Dunn, S. M., Gnesin, S., & Prior, J. O. (2021). Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics, 13(1), 96. https://doi.org/10.3390/pharmaceutics13010096