Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hot-Melt Extrusion (HME)
2.2. Dissolution Testing
2.3. Side-by-Side Diffusion Cell
2.4. Polarized Light Microscopy (PLM)
2.5. Particle Size and Zeta Potential Analysis
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Solid-State 13C Nuclear Magnetic Resonance (NMR) (ssNMR) Spectroscopy
2.8. Solution 1H NMR Spectroscopy
2.9. Animal Studies
2.10. Statistical Analyses
3. Results
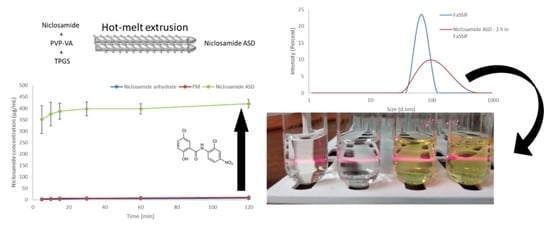
3.1. Hot-Melt Extrusion Successfully Prepared Amorphous Extrudates of Niclosamide
3.2. The Amorphous Extrudates Increased Niclosamide’s Apparent Solubility
3.3. The Amorphous Extrudates Increased Niclosamide Diffusion in Side-by-Side Diffusion Cells
3.4. FTIR
3.5. Solid-State NMR Shows the Importance of the Phenolic Group in the Amorphous Dispersion
3.6. Solution NMR Showed the Relevance of the 2-Pyrrolidinone Group for Niclosamide’s Stabilization after Dissolution
3.7. pH Shift Dissolution Testing Confirms that the Amorphous Extrudates Crystallize in Acidic Conditions
3.8. The Amorphous Extrudates Increased the Bioavailability of Niclosamide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, E.J.; Löbenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide Repositioning for Treating Cancer: Challenges and Nano-Based Drug Delivery Opportunities. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2019, 141, 58–69. [Google Scholar] [CrossRef] [PubMed]
- WHO Model List of Essential Medicines. Available online: https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06 (accessed on 3 January 2021).
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.-K.; Roberts, M.J.; Arend, R.C.; Samant, R.S.; Buchsbaum, D.J. Multi-Targeted Therapy of Cancer by Niclosamide: A New Application for an Old Drug. Cancer Lett. 2014, 349, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an Antihelminthic Drug. Cell. Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Hamza, T.; Ma, B.; Chen, K.; Beilhartz, G.L.; Ravel, J.; Feng, H.; Melnyk, R.A. Host-Targeted Niclosamide Inhibits C. Difficile Virulence and Prevents Disease in Mice without Disrupting the Gut Microbiota. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Alzghoul, A.; Kaialy, W.; Mahlin, D.; Bergström, C.A.S. Computational Predictions of Glass-Forming Ability and Crystallization Tendency of Drug Molecules. Mol. Pharm. 2014, 11, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Kumar, S.S.; Nangia, A. Pharmaceutical Cocrystals of Niclosamide. Cryst. Growth Des. 2012, 12, 4588–4599. [Google Scholar] [CrossRef]
- van Tonder, E.C.; Maleka, T.S.P.; Liebenberg, W.; Song, M.; Wurster, D.E.; de Villiers, M.M. Preparation and Physicochemical Properties of Niclosamide Anhydrate and Two Monohydrates. Int. J. Pharm. 2004, 269, 417–432. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A Phase I Study of Niclosamide in Combination with Enzalutamide in Men with Castration-Resistant Prostate Cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [Green Version]
- Grifasi, F.; Chierotti, M.R.; Gaglioti, K.; Gobetto, R.; Maini, L.; Braga, D.; Dichiarante, E.; Curzi, M. Using Salt Cocrystals to Improve the Solubility of Niclosamide. Cryst. Growth Des. 2015, 15, 1939–1948. [Google Scholar] [CrossRef]
- Luedeker, D.; Gossmann, R.; Langer, K.; Brunklaus, G. Crystal Engineering of Pharmaceutical Co-Crystals: “NMR Crystallography” of Niclosamide Co-Crystals. Cryst. Growth Des. 2016, 16, 3087–3100. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, M.A.; Khan, W.S.; Shafique, M.; Khan, M. Fabrication of Niclosamide Loaded Solid Lipid Nanoparticles: In Vitro Characterization and Comparative in Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1926–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Yao, Y. Octenylsuccinate Hydroxypropyl Phytoglycogen Enhances the Solubility and In-Vitro Antitumor Efficacy of Niclosamide. Int. J. Pharm. 2018, 535, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-Targeted Pluronic® P123/F127 Mixed Micelles Delivering Niclosamide: A Repositioning Strategy to Treat Drug-Resistant Lung Cancer Cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Costabile, G.; d’Angelo, I.; Rampioni, G.; Bondì, R.; Pompili, B.; Ascenzioni, F.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Miro, A.; et al. Toward Repositioning Niclosamide for Antivirulence Therapy of Pseudomonas Aeruginosa Lung Infections: Development of Inhalable Formulations through Nanosuspension Technology. Mol. Pharm. 2015, 12, 2604–2617. [Google Scholar] [CrossRef]
- Naqvi, S.; Mohiyuddin, S.; Gopinath, P. Niclosamide Loaded Biodegradable Chitosan Nanocargoes: An in Vitro Study for Potential Application in Cancer Therapy. R. Soc. Open Sci. 2017, 4, 170611. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Zhang, T.; Zhang, J.; Wu, B. Significantly Enhanced Bioavailability of Niclosamide through Submicron Lipid Emulsions with or without PEG-Lipid: A Comparative Study. J. Microencapsul. 2015, 32, 496–502. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Zhang, T.; Wang, H.; Wu, B. Design and Evaluation of Injectable Niclosamide Nanocrystals Prepared by Wet Media Milling Technique. Drug Dev. Ind. Pharm. 2015, 41, 1416–1424. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of Increased Bioavailability through Amorphous Solid Dispersions: A Review. Drug Deliv. 2019, 27, 110–127. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Lou, X.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Mechanism of Ritonavir—Copovidone Amorphous Solid Dispersions: Importance of Congruent Release for Enhanced Performance. Mol. Pharm. 2019, 16, 1327–1339. [Google Scholar] [CrossRef]
- Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic Screening of Pharmaceutical Polymers for Hot Melt Extrusion Processing: A Comprehensive Review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Bulduk, B.; Lindenberg, E.; Löbmann, K.; Rades, T.; Grohganz, H. Influence of Glass Forming Ability on the Physical Stability of Supersaturated Amorphous Solid Dispersions. J. Pharm. Sci. 2019, 108, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Grohganz, H.; Lindenberg, E.; Löbmann, K.; Müllertz, A.; Rades, T. The Influence of Polymers on the Supersaturation Potential of Poor and Good Glass Formers. Pharmaceutics 2018, 10, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Is There a Correlation between the Glass Forming Ability of a Drug and Its Supersaturation Propensity? Int. J. Pharm. 2018, 538, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ferreira, R.; Zhang, F. Effect of Surfactant Level on Properties of Celecoxib Amorphous Solid Dispersions. J. Drug Deliv. Sci. Technol. 2019, 49, 301–307. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Understanding the Generation and Maintenance of Supersaturation during the Dissolution of Amorphous Solid Dispersions Using Modulated DSC and 1H NMR. Int. J. Pharm. 2018, 536, 414–425. [Google Scholar] [CrossRef]
- Ray, E.; Vaghasiya, K.; Sharma, A.; Shukla, R.; Khan, R.; Kumar, A.; Verma, R.K. Autophagy-Inducing Inhalable Co-Crystal Formulation of Niclosamide-Nicotinamide for Lung Cancer Therapy. AAPS PharmSciTech 2020, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- van Tonder, E.C.; Mahlatji, M.D.; Malan, S.F.; Liebenberg, W.; Caira, M.R.; Song, M.; de Villiers, M.M. Preparation and Physicochemical Characterization of 5 Niclosamide Solvates and 1 Hemisolvate. AAPS PharmSciTech 2004, 5, E12. [Google Scholar] [CrossRef]
- Worku, Z.A.; Aarts, J.; Singh, A.; Van den Mooter, G. Drug—Polymer Miscibility across a Spray Dryer: A Case Study of Naproxen and Miconazole Solid Dispersions. Mol. Pharm. 2014, 11, 1094–1101. [Google Scholar] [CrossRef]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.G.M.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and Evaluation of Cyclodextrin Complexes for Improved Anticancer Activity of Repurposed Drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef]
- Song, Y.; Yang, X.; Chen, X.; Nie, H.; Byrn, S.; Lubach, J.W. Investigation of Drug–Excipient Interactions in Lapatinib Amorphous Solid Dispersions Using Solid-State NMR Spectroscopy. Mol. Pharm. 2015, 12, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Egami, K.; Higashi, K.; Yamamoto, K.; Moribe, K. Crystallization of Probucol in Nanoparticles Revealed by AFM Analysis in Aqueous Solution. Mol. Pharm. 2015, 12, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Que, C.; Lou, X.; Zemlyanov, D.Y.; Mo, H.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Behavior of Ledipasvir–Copovidone Amorphous Solid Dispersions: Role of Drug Loading and Intermolecular Interactions. Mol. Pharm. 2019, 16, 5054–5067. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Taylor, L.S. Polymer Type Impacts Amorphous Solubility and Drug-Rich Phase Colloidal Stability: A Mechanistic Study Using Nuclear Magnetic Resonance Spectroscopy. Mol. Pharm. 2020, 17, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Sato, K.; Fukushima, M.; Miyazaki, A.; Yamamura, Y.; Sakuma, S. Phase Separation of Supersaturated Solution Created from Amorphous Solid Dispersions: Relevance to Oral Absorption. Eur. J. Pharm. Biopharm. 2018, 132, 146–156. [Google Scholar] [CrossRef]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of Rat and Mouse Gastrointestinal PH, Fluid and Lymphoid Tissue, and Implications for in-Vivo Experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef]
- Palmelund, H.; Madsen, C.M.; Plum, J.; Müllertz, A.; Rades, T. Studying the Propensity of Compounds to Supersaturate: A Practical and Broadly Applicable Approach. J. Pharm. Sci. 2016, 105, 3021–3029. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Shen, C.; Shen, B.; Zhong, R.; Yuan, H. Effect of Particle Size on in Vitro and in Vivo Behavior of Astilbin Nanosuspensions. J. Drug Deliv. Sci. Technol. 2019, 52, 778–783. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Wang, N.; Shen, P.; Dou, H.; Ma, K.; Gao, Y.; Zhang, J.; Qian, S. Mechanistic Study on Complexation-Induced Spring and Hover Dissolution Behavior of Ibuprofen-Nicotinamide Cocrystal. Cryst. Growth Des. 2018, 18, 7343–7355. [Google Scholar] [CrossRef]
- Harmon, P.; Galipeau, K.; Xu, W.; Brown, C.; Wuelfing, W.P. Mechanism of Dissolution-Induced Nanoparticle Formation from a Copovidone-Based Amorphous Solid Dispersion. Mol. Pharm. 2016, 13, 1467–1481. [Google Scholar] [CrossRef]
- Stewart, A.M.; Grass, M.E.; Brodeur, T.J.; Goodwin, A.K.; Morgen, M.M.; Friesen, D.T.; Vodak, D.T. Impact of Drug-Rich Colloids of Itraconazole and HPMCAS on Membrane Flux in Vitro and Oral Bioavailability in Rats. Mol. Pharm. 2017, 14, 2437–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricarte, R.G.; Van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Denninger, A.; Westedt, U.; Rosenberg, J.; Wagner, K.G. A Rational Design of a Biphasic Dissolution Setup—Modelling of Biorelevant Kinetics for a Ritonavir Hot-Melt Extruded Amorphous Solid Dispersion. Pharmaceutics 2020, 12, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tho, I.; Liepold, B.; Rosenberg, J.; Maegerlein, M.; Brandl, M.; Fricker, G. Formation of Nano/Micro-Dispersions with Improved Dissolution Properties upon Dispersion of Ritonavir Melt Extrudate in Aqueous Media. Eur. J. Pharm. Sci. 2010, 40, 25–32. [Google Scholar] [CrossRef]
- Klumpp, L.; Leigh, M.; Dressman, J. Dissolution Behavior of Various Drugs in Different FaSSIF Versions. Eur. J. Pharm. Sci. 2020, 142, 105138. [Google Scholar] [CrossRef]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; et al. Nanocrystal-Based per-Oral Itraconazole Delivery: Superior in Vitro Dissolution Enhancement versus Sporanox® Is Not Realized in in Vivo Drug Absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Yeh, T.-K.; Lin, K.-T.; Chen, W.-C.; Yao, H.-T.; Lan, S.-J.; Wu, Y.-S.; Hsieh, H.-P.; Chen, C.-M.; Chen, C.-T. Pharmacokinetics of Anti-SARS-CoV Agent Niclosamide and Its Analogs in Rats. J. Food Drug Anal. 2006, 14, 6. [Google Scholar]
- Bergström, C.A.S.; Wassvik, C.M.; Johansson, K.; Hubatsch, I. Poorly Soluble Marketed Drugs Display Solvation Limited Solubility. J. Med. Chem. 2007, 50, 5858–5862. [Google Scholar] [CrossRef]
- Pardhi, V.; Chavan, R.B.; Thipparaboina, R.; Thatikonda, S.; Naidu, V.; Shastri, N.R. Preparation, Characterization, and Cytotoxicity Studies of Niclosamide Loaded Mesoporous Drug Delivery Systems. Int. J. Pharm. 2017, 528, 202–214. [Google Scholar] [CrossRef]








| PK Parameters | Niclosamide Anhydrate Suspension in FaSSIF | Niclosamide ASD Suspension in FaSSIF | Niclosamide ASD in Capsules |
|---|---|---|---|
| T ½ (h) | 1.00 (0.3.0) | 1.59 (1.34) | 0.84 (0.01) |
| T max (h) | 3.60 (0.89) | 2.40 (1.52) | 4.40 (0.89) |
| C max (ng/mL) | 48.3 (20.6) | 123 (56) | 122 (71) |
| AUC last (h × ng/mL) | 168 (64) | 398 (115) | 338 (193) |
| AUC Inf (h × ng/mL) | 188 (84) | 495 (239) | 463 (224) |
| AUC_%Extrap_obs (%) | 8.4 (7.0) | 12.2 (21.4) | 7.98 (0.48) |
| MRT Inf_obs (%) | 3.56 (0.70) | 3.71 (2.20) | 4.04 (0.12) |
| AUC last/D (h × mg/mL) | 16.8 (6.4) | 39.8 (11.5) | 33.8 (19.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara, M.O.; Warnken, Z.N.; Williams, R.O., III. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 2021, 13, 97. https://doi.org/10.3390/pharmaceutics13010097
Jara MO, Warnken ZN, Williams RO III. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics. 2021; 13(1):97. https://doi.org/10.3390/pharmaceutics13010097
Chicago/Turabian StyleJara, Miguel O., Zachary N. Warnken, and Robert O. Williams, III. 2021. "Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study" Pharmaceutics 13, no. 1: 97. https://doi.org/10.3390/pharmaceutics13010097
APA StyleJara, M. O., Warnken, Z. N., & Williams, R. O., III. (2021). Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics, 13(1), 97. https://doi.org/10.3390/pharmaceutics13010097