1. Introduction
The blood–brain barrier (BBB) is the most important exchange surface between the blood and the central nervous system (CNS). As the CNS requires a precise and balanced microenvironment to work efficiently, this physical border enables cerebral homeostasis, which regulates the very selective passage of molecules. Microvascular endothelial cells, which constitute the BBB, have specific characteristics, such as the expression of specific transporters causing a selective transcellular passage, a low proportion of transport vesicles (compared to other endothelia), thus limiting the passage of molecules with high molecular weight, and the presence of tight junctions with high electrical resistance [
1]. This BBB function is generally considered to be a protective mechanism against virus and unwanted substances circulating in the blood and to control the flow of substances required for brain function [
2]. However, BBB failures are described in numerous diseases of the CNS with neurodegenerative, inflammatory, infectious, or even neoplastic components. These dysfunctions can be either seen as a consequence of disease progression or can be involved in the early pathophysiology of the disease (e.g., multiple sclerosis). The consequence is a change in the permeability of the BBB and the delivery of substances from the vascular circulation to the brain or cellular infiltration across the BBB. A better understanding of the mechanisms controlling BBB permeability will help to target BBB interventions that could have therapeutic interests, such as increasing drug delivery to the brain or reducing the damaging effects of inflammation or waste accumulation.
Among the substances that have been studied for their ability to modulate cerebral blood flow and BBB permeability, serotonin (5-HT) has been proposed by several authors [
3]. The suggested mechanism is based on an interaction between astrocytes and endothelial cells constituting the BBB. For these authors, cerebral 5-HT acting on astrocytes could lead to the synthesis of ATP and prostaglandins with effects on vascular tone and endothelial permeability [
3]. On the other hand, platelets transport 5-HT in the blood and constitute the main store of peripheral 5-HT. During epileptic seizures, it was demonstrated that platelets degranulation modulates BBB permeability and increases cerebral 5-HT concentration [
4]. However, a direct role of 5-HT in modulating BBB permeability has not yet been demonstrated. Furthermore, only a few publications have reported that microvascular endothelial cells express mRNA encoding for 5-HT receptors, 5-HT
1D and 5-HT
7 being the most extensively studied, although their functional role in the permeability of the BBB has not been investigated [
5].
The 5-HT
4 receptor is highly expressed in neurons of the olfactory bulb, islands of Calleja, basal ganglia, nucleus accumbens, hippocampus, and substantia nigra [
6,
7,
8]. It is co-localized on cholinergic (stimulation of acetylcholine release), glutamatergic and spiny neurons [
9]. The 5-HT
4 receptor is also expressed in intrinsic primary afferent neurons, motor neurons and enterocytes of the gut, in the myocytes of the atria (increased expression in ventricles of heart failing patients) and on the surface of cholinergic and purinergic neurons innervating the detrusor muscle [
10]. Finally, its expression is also found in the zona glomerulosa and zona fasciculata of the adrenal gland cortex [
11,
12].
The 5-HT
4 receptor is a G protein coupled receptor positively coupled to adenylate cyclase in neurons and enterocytes. Protein kinase A activation leads to the inhibition of voltage-gated K
+ channels, including Ca
2+ activated K
+ channels, produces a long-lasting inhibition of K
+ channels and activates or inhibits GABAergic synaptic transmission [
13]. In cardiac myocytes, 5-HT
4 receptors activate L-type currents via PKA. After 5-HT
4 receptors stimulation, cAMP directly activates hyperpolarization-activated current I
h. These receptors also activate the exchange factor “Exchange Protein Activated by cAMP”, which is a Rap guanine nucleotide exchange factor [
14]. Another coupling pathway is described in primary neurons, where the 5-HT
4 receptor activates the extracellular regulated kinase (ERK) pathway in a G(s)/cAMP/PKA independent manner [
15]. This last effect appears to be dependent on the Src tyrosine kinase [
15].
In this study, we demonstrate the presence of the 5-HT4 receptor in human and rat microvascular endothelial cells. Further, hCMEC/D3 cells were used as a cellular model of the BBB, and we hypothesized that 5-HT4 receptor stimulation could induce a change in BBB permeability. Therefore, we initiated a study dedicated to characterize the functional roles of this receptor and the signaling pathway stimulated by prucalopride, a 5-HT4 receptor agonist, in our cellular BBB model. Finally, the role of the 5-HT4 receptor in modifying BBB permeability was studied in prucalopride treated rats.
2. Materials and Methods
2.1. Materials
The human cerebral microvascular endothelial cells (hCMEC) line, D3 clone (hCMEC/D3; #CLU512-A), were purchased from Tebu-bio company (Le Perray-en-Yvelines, France). Products purchased from Thermo Fisher Scientific (Illkirch-Graffenstaden, France) were as follows: chemically defined lipid concentrate (# 11905031), fetal bovine serum (#10270-106), penicillin–streptomycin (#15070-063), HEPES (#15630-080), TRIzol™ reagent (#15596026), FITC dextran (3 kDa) (#D3305), FITC dextran (10 kDa) (#D1821) and competitive immunoassay kit for the quantification of cyclic AMP (cAMP) (#EMSCAMPL).
The chemicals purchased from Sigma-Aldrich (St. Quentin Fallavier, France) were hydrocortisone (#H0135), ascorbic acid (#A4544), βFGF (#F0291), prucalopride (#SML-1371), GR113808 (#G5918), dimethyl sulfoxide (#D5879), PP2 (#P0042), Trypan blue (#T8154), blue dextran (5 kDa) (#90008) and CD31 monoclonal anti-rabbit antibodies (clone EP78, monoclonal). H89 dihydrochloride (#2910) was purchased from Tocris (Noyal Châtillon sur Seiche, France). The iScript™ RT-qPCR Sample Preparation Reagent, the SYBR Green PCR Master Mix and the PCR primers of 5-HT
4 receptor and 18S were obtained from Bio-Rad (Marnes-la-Coquette, France) and described in
Table S1, Supplementary Materials. PhosSTOP™ (#4906845001) and cOmplete™ ULTRA Tablets (#5892970001) were obtained from Roche (Basel, Switzerland). Specific primary antibodies against 5-HT
4 receptor (#ab60359) were purchased from Abcam (Cambridge, the United Kingdom). Occludin (#PA5-20755) and ZO-1 (#33-9100) antibodies were obtained from Thermo Fisher Scientific. ERK1/2 (#9102S) and Phospho ERK1/2 (#04797) were obtained from Cell Signaling (Leiden, Netherlands) and Merck Millipore (Molsheim, France), respectively. Claudin-5 (#E-AB-30946) antibody was obtained from Elabscience (distributor Euromedex, Souffelweyersheim, France). Alexa-488 and -563 fluorophore-conjugated secondary antibodies were purchased from Merck Millipore. Goat serum (#S2000-100) was obtained from Dutscher (Bernolsheim, France). Luminata, the ECL reagent, was obtained from Merck Millipore. Vectashield mounting medium containing DAPI (#H-1200), the anti-rabbit biotinylated secondary antibody (#BA-1100), ABC amplification kit (#PK-6100) and VIP substrate (#SK-4600) were purchased from Vector Laboratories (Burlingame, CA, USA).
The 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (#ab197010), the Protein Kinase A (PKA) activity kit (#ab139435) and phosphorylated Src and total Src kit (#ab207461) were obtained from Abcam.
2.2. Cell Culture
Human cerebral microvascular endothelial cells (hCMEC/D3) were maintained in endothelial basal medium 2 (EBM-2) as previously described [
16]. All culture media were supplemented with chemically defined lipid concentrate (1%), fetal bovine serum (5%), penicillin–streptomycin (1%), hydrocortisone (1.4 µM), ascorbic acid (5 µg·mL
−1), HEPES (10 mM) and βFGF (1 ng·mL
−1). Tebu-bio provided a vial of this cell line with a passage number between 25 and 27. Cells were cultured and passed twice a week in a 75 cm
2 flask.
2.3. Cells Treatments
Cells were seeded at 2.2 × 106 cells per cm2, on different supports depending on the experiment. Prucalopride and GR113808 were dissolved in dimethyl sulfoxide to obtain 1 mM stock solutions and diluted in the culture medium prior to cell treatment. Endothelial cells were treated for 96 h with prucalopride (10 µM), GR113808 (1 µM) or the combination of prucalopride (10 µM) and GR113808 (1 µM). All cells were depleted of fetal bovine serum at day 3 after the start of treatment, while the treatment was continued.
2.4. RNA Extraction and Real Time qPCR
Total RNA was extracted from hCMEC/D3 using the Invitrogen™ TRIzol™ reagent. RNA concentration was quantified by the NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Furthermore, 200 nanograms of RNA was extracted to synthesize cDNAs in a thermal cycler with the iScript™ RT-qPCR Sample Preparation Reagent, and then PCR was performed with the SYBR Green PCR Master Mix using a CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). The relative amount in each sample was normalized to the level of expressed 18S mRNA.
2.5. Western Blot
After treatment, the cells were lysed with lysis buffer supplemented with protease and phosphatase inhibitors, cells extracts were collected and centrifuged for 10 min at 10,000×
g at 4 °C. Total cell lysates containing 10 µg of protein were separated using 10% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane. Non-specific binding sites were blocked with 10% non-fat milk in phosphate buffered saline (PBS) with 0.1% Tween 20 detergent. Membranes were incubated with primary antibodies (5-HT
4 receptor, ZO-1, occludin, claudin-5, ERK1/2, and Phospho-ERK1/2) overnight at 4 °C. Membranes were extensively washed and then incubated for 1 h with secondary antibodies at room temperature. After another extensive wash, protein bands were visualized by Luminata, ECL reagent, and using the ChemiDoc XRS+ detection system (Bio-Rad). Proteins extracted from human neuroblastoma cell line (SH-SY5Y cell line) were used as a positive control of 5-HT
4 receptor protein expression [
17], and proteins extracted from lung papillary adenocarcinoma (NCI-H441 cell line) were used as a negative control of 5-HT
4 receptor protein expression.
The relative intensity was densitometrically determined by using the Image Lab software (Biorad) as the intensity of the bands relative to the total protein ratio revealed by the stain free method. The stain free imaging technology uses a proprietary polyacrylamide gel chemistry to make proteins fluorescent directly in the gel with a short photoactivation, allowing immediate visualization of proteins at any point during electrophoresis and blotting. This method eliminates the inherently problematic use of housekeeping proteins as loading controls on Western blots, permitting the user to obtain truly quantitative Western blot data by normalizing the bands to the total protein in each lane.
2.6. Cells Immunofluorescence Assay
Cells were seeded on glass coverslips, fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature. Cells were permeabilized with Triton X-100 at 0.1% and blocked with 10% goat serum in PBS. After a first incubation with rabbit polyclonal anti-5-HT4 receptor primary antibody (1:1000) at 4 °C overnight, cells were extensively washed and then incubated with Alexa 488 fluorophore-conjugated secondary antibodies (1:1000) for 4 h. After another wash, coverslips were mounted, and images were obtained with a Leica TCS SP5 MP confocal microscope.
2.7. Histological Analysis
In rats, PBS was infused via the left ventricle to initiate rinsing the vasculature and remove blood components. This was followed by 4% PFA in 0.1 M PBS infusion. Brains were fixed by immersion in PFA. After 48 h, the tissue was dehydrated in a series of ethanol solutions of increasing concentration followed by immersion in toluene. Brains were finally placed in liquid paraffin to form a “paraffin block”.
Immunostaining was performed in formalin-fixed, paraffin-embedded rat CNS sections. Thick tissue slices (3–5 µm) were cut using a microtome. After deparaffinization, tissue rehydration and inactivation of endogenous peroxidase, the sections were subjected to the antigen retrieval process (1:1000 rabbit polyclonal anti-5-HT4 receptor) at 4 °C overnight, followed by a blocking step to remove non-specific binding sites. The 5-HT4 receptor was visualized by peroxidase reaction products.
Paraffin-embedded human brain tissue was analyzed. For this, 5 µm-thick sections were deparaffinized and antigen microwave retrieval in 0.01 M sodium citrate buffer (pH 6) was performed (10 min, 850 W) followed by sequential incubation with rabbit polyclonal anti-5-HT4 (1:100 dilution) and Alexa 488-anti-rabbit secondary antibody (1:400 dilution), then with rabbit monoclonal anti-CD31 (1:50) followed by Alexa 568 anti-rabbit secondary antibody (1:400 dilution). Incubation with primary antibodies was performed overnight; secondary antibodies were incubated for 1 h. Extensive washing in 0.01 M Tween was performed between each incubation step. After washing, slides were mounted in Vectashield mounting medium containing DAPI. The specificity of the secondary antibodies was controlled by switching the correspondence with the primary antibodies and by replacing primary antibodies with non-specific immunoglobulins. The 5-HT4 immunostaining was equally performed using an anti-rabbit biotinylated secondary antibody, ABC amplification kit and VIP substrate.
2.8. Trans-Endothelial Electric Resistance and Permeability Assay
Cells were seeded at 1 × 106 cells on polyethylene terephthalate inserts with 0.4 µm pore size and 4.5 cm2 area (#833930041, Sarstedt, Nümbrecht, Germany) previously coated with a thin layer of collagen I. The hCMEC/D3 cells cultured at 37 °C in 5% CO2 constituted our in vitro BBB model. Trans-endothelial electric resistance (TEER) indicating the paracellular permeability of the hCMEC/D3 monolayer was measured with the Evom2® epithelial Ohm meter (World Precision Instruments, Sarasota, FL, USA). The inserts were placed in a cell culture cup chamber for TEER measurement with EndOhm® containing a pair of concentric electrodes. An insert without hCMEC/D3 cells, used as a blank control, was measured and subtracted from the TEER values measured with hCMEC/D3 cells. The result was multiplied by the total membrane surface area to obtain the resistance value in Ω·cm2. Measurements were performed daily for 4 days after the start of prucalopride, GR113808 or prucalopride + GR113808 treatments. Kinetics of paracellular permeability were assessed by the area under the curve of TEER (AUCTEER) measurement during the 96 h of treatment. When AUCTEER decreased, the hCMEC/D3 barrier permeability increased.
To assess the size of pores that allowed paracellular permeability of the hCMEC/D3 cell monolayer, we used molecules of various molecular weights. Trypan blue (2.9 × 10−1 mM, 0.87 kDa), blue dextran (2 mM, 5 kDa) or FITC dextran (10 µM, 3 kDa and 10 µM, 10 kDa) were added in the upper chamber, and their cell monolayer crossing was assessed by reading the absorbance in the bottom chamber. A percentage was obtained as a ratio of absorbance at 595 nm or fluorescence at 490/520 nm, 8 h after the addition of markers in the upper chamber (control set at 100%). When blue dyes were used, samples were analyzed using the iMark™ microplate absorbance reader. Fluorescein was analyzed with a Xenius XM microplate spectrofluorometer reader (Safas, Monaco). The results were expressed as permeability flux percentages.
2.9. MTS Assay
The MTS assay was used to evaluate cell proliferation and viability after prucalopride treatment. The hCMEC/D3 cells were seeded in 96-well culture plates in culture medium at 37 °C for 96 h in the presence or absence of prucalopride (10 µM). The viability was estimated using MTS reduction to the formazan dye. MTS was added to cells and incubated for 2 h. Then, the absorbance of medium containing formazan was measured at 490 nm. The results were expressed as a percentage of viability, compared to the control conditions arbitrarily set at 100%.
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
Competitive immunoassay was performed for the relative quantification of cAMP. The PKA activity analysis was performed with an enzyme-linked immuno-absorbent assay that utilizes a specific synthetic peptide as a substrate for PKA and a polyclonal antibody that recognizes the phosphorylated form of the substrate. Phosphorylated Src and total Src were quantified with an ELISA kit. All these kits were used according to manufacturer’s instructions.
2.11. Evans Blue Diffusion to the Brain
BBB permeability in rats was evaluated by the cerebral diffusion of Evans blue after prucalopride or vehicle treatment. All animal care and procedures were in accordance with institutional guidelines and European regulations. The protocol was adapted from Goldim et al. and submitted to the French regulatory authorities and ethics committee (registration number: 26564-202007101135885) [
18]. Two groups of 5 adult Wistar rats (control group 301.4 ± 35.84 g; prucalopride group 343.6 ± 36.54 g) (Wistar Han IGS, Charles River, Wilmington, MA, USA) were treated with either daily intraperitoneal injection of 10 mg·kg
−1 prucalopride or vehicle (10% DMSO in NaCl 0.9%). The animals were monitored in order to detect weight lost, signs of dehydration or diarrhea and each measurable discomfort. Furthermore, 96 h after the start of treatment, 3 mL·kg
−1 of 2% Evans blue was infused in the left ventricle immediately followed by 10 mL·kg
−1 of PBS. The brain slices were snaped with a Leica macroscope (M651). Brain samples were homogenized, using a tissue grinder containing 250 µL of 50% orthophosphoric acid per gram of sample, and absorbance at 595 nm (iMark™ microplate absorbance reader, Bio-Rad) was used for a quantitative assessment of blue dye content in the brain parenchyma.
2.12. Statistical Analysis
Values were mean ± SEM from three or more independent experiments. Data were reported as mean ± SEM, and p < 0.05 was considered statistically significant. Differences were analyzed using a Student’s t-test or one-way ANOVA with GraphPad Prism 6 (GraphPad, San Diego, CA, USA).
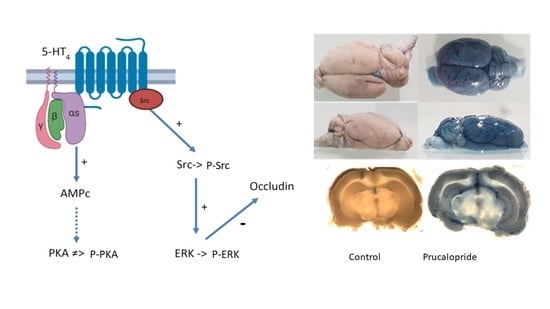
4. Discussion
In this study, through the use of the 5-HT
4 receptor agonist prucalopride, we investigated the role of the 5-HT
4 receptor in the modulation of BBB permeability. For the first time, 5-HT
4 receptor expression was described in the hCMEC/D3 cell line, a well-known in vitro BBB model, but also in human and rat brain microvascular cells [
20]. Our second goal was to investigate how prucalopride regulates the paracellular permeability of hCMEC/D3 cells. The 5-HT
4 receptor stimulation led to a decrease in the TEER of BBB cells model and increased the passage of Trypan blue (0.87 kDa) and FITC dextran (3 kDa) but not blue dextran (5 kDa) and FITC dextran (10 kDa) to the brain. On the other hand, the blockade of the 5-HT
4 receptor by GR113808, a selective 5-HT
4 receptor antagonist, prevented the decrease in TEER and the permeability for Trypan blue and FITC dextran (3 kDa). Furthermore, we found that prucalopride affected the expression of the tight junction protein, occludin. Immunoblot experiments revealed that the expression of occludin in cells treated with prucalopride was inferior to that of the controls. However, ZO-1 and claudin-5 tight junction proteins expression was not directly affected by prucalopride. Intercellular tight junctions exhibit a complex molecular architecture involving integral membrane linker proteins, such as occludin and claudins and cytoplasmic adaptor proteins. Occludin, a 65 kDa tetraspan protein, is a key functional component of the tight junction stabilization that plays a critical role in maintaining BBB properties. Occludin C-terminal cytoplasmic domain is required for binding the ZO-1 GLUK domain and the cytoskeleton [
21]. Consequently, occludin downregulation could impact the functional role of other tight junction proteins. This phenomenon could explain the tendency to increase the protein expression of ZO-1 observed in our in vitro model after treatment with prucalopride. Some research reported that epithelial barriers are functional in rodents carrying a null mutation of the gene coding for occludin [
22]. However, the insertion of an occludin mutant or the null mutation of a gene encoding occludin cannot be compared to the acute effects of prucalopride treatment leading to the downregulation of occludin in a constituted barrier.
The 5-HT
4 receptor is a G-protein-coupled receptor positively coupled to adenylate cyclase in neurons and enterocytes [
13]. In primary neurons, the 5-HT
4 receptor activates the ERK pathway in a G(s)/cAMP/PKA-independent manner [
15]. The 5-HT
4 receptor-mediated ERK activation is dependent on Src, a nonreceptor tyrosine kinase [
15]. Therefore, we investigated the signaling pathways involved in the occludin-mediated permeability variations. Surprisingly, prucalopride moderately improved cAMP production in hCMEC/D3 and did not activate PKA. On the other hand, prucalopride induced Src and ERK1/2 activation. Src kinase has already been described as being involved in occludin regulation. In transient focal cerebral ischemia, Takenaga et al. reported that the inhibition of Src activity decreases the tyrosine phosphorylation of occludin in brain capillaries and attenuates increased permeability of the BBB [
23]. Moreover, Zhang et al. described that propofol attenuates the tumor necrosis factor-α induced occludin downregulation by inhibiting ERK1/2 in the hCMEC/D3 cell line [
24]. The 5-HT
4 receptor is a constitutively coupled Gs receptor. According to Liu et al., it can be hypothesized that constitutive activated Gs protein is critical to initiate ERK1/2 activation and that another ligand-induced pathway activation is responsible for the amplification and maintenance of ERK signaling [
25].
The microvascular brain endothelial cells restrict blood–brain exchanges. However, this barrier is finely regulated by the surrounding environment, namely, the neurovascular unit. Astrocytes are particularly described for regulating the permeability of BBB [
3]. Consequently, co-culture models, including astrocytes, are frequently proposed [
26]. However, the characteristics of this heterogeneous lineage depend on the brain structure of origin. In vitro models make complex the discrimination of cellular mechanisms localized in endothelial and other cell types. Although their roles are less studied, other cell lines appear to influence the permeability of the BBB (as pericytes or microglial cells). Thus, in vitro modeling of the BBB cannot be extrapolated in vivo; this is why new animal experiments are necessary. In this work, rather than developing complex coculture to model BBB, we decided to confirm the in vitro results by animal experiments. The quantitative assessment of BBB permeability was performed by measuring the passage of Evans blue in brain structures, a method widely described in the literature [
18]. The administration of Evans blue after 4 days of treatment with prucalopride (10 mg·kg
−1 per day) confirmed the effect of prucalopride on BBB permeability. Barthet et al. reported that 5-HT
4 receptor mediated ERK activation is transient and that 5-HT
4 receptor colocalizes with Src even after the receptor endocytosis [
15]. Given that rats were sacrificed, we could not assess the duration of BBB opening after the end of treatment. Unlike many studies that assessed the permeabilization of BBB in a localized area of the brain, a localized effect of prucalopride was not expected. Evans blue is homogenously distributed in neuron-rich areas but appears to diffuse less in the white matter, favoring the hypothesis of the heterogeneity of BBB across brain structures [
27]. Future work should focus on the origin of 5-HT involved in the modulation of BBB permeability mediated by the 5-HT
4 receptor. It could be of a peripheral origin and therefore, coming from platelet degranulation (platelets contain about 95% of the 5-HT of the body). A central origin of 5-HT for the effects observed in this study cannot be fully ruled out.
The 5-HT4 receptor is a potential target to develop new pharmacological strategies aiming to open the BBB. Some drugs developed in neurodegenerative or brain tumor diseases show poor cerebral diffusion and require high posology to achieve therapeutic concentrations in the brain. These high dosages may cause peripheral adverse events, poor tolerance or safety issues. New pharmacological strategies that open the BBB without disrupting it are required. These active substances targeting the 5-HT4 receptor could transiently enhance brain distribution and consequently improve the efficacy and safety of several drugs.