Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine
Abstract
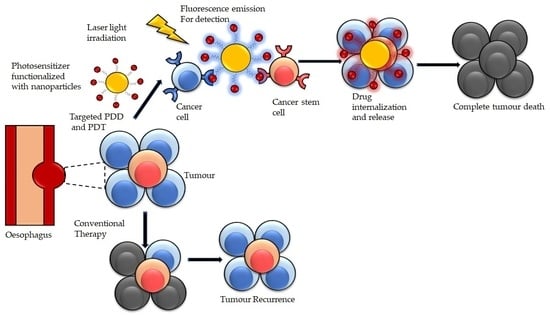
:1. Introduction
2. Classic Diagnosis of Esophageal Cancer
3. Classic Treatment of Esophageal Cancer
4. Photodynamic Diagnosis (PDD) and Therapy (PDT) for Esophageal Cancer
4.1. Principle of Photodynamic Diagnosis and Photodynamic Therapy
4.2. Characteristics of an Ideal Photosensitizer for Photodynamic Diagnosis and Photodynamic Therapy
4.3. Photosensitizer for Esophageal Cancer PDD Applications
4.4. Photosensitizer for Esophageal Cancer PDT Applications
4.5. Limitations and Strategies to Improve Photodynamic Diagnosis and Photodynamic Therapy
4.6. Photodynamic Therapy in Combination with Conventional Therapy
4.7. Targeted Photodynamic Diagnosis and Photodynamic Therapy
5. Photoimmunotherapy for Targeted Photodynamic Diagnosis and Photodynamic Therapy
6. Nanomedicine for Targeted Photodynamic Diagnosis and Photodynamic Therapy
6.1. Nanomedicine Platform for Targeted Photodynamic Diagnosis and Photodynamic Therapy
| Inorganic NP Platforms | Characteristics | References |
|---|---|---|
| Gold NPs | Allows for surface functionalization Excellent optical/photoresponsive feature Good biocompatibility Minimal toxicity High ROS production Enhance cancer cell destruction Improve drug delivery target site surface Plasmon resonance (LSPR) characteristics | [73,77,88,89] |
| Silver NPs | Brilliant optical and physiochemical features High production of ROS High surface volume to ratio Easy surface modification Enhance anti-tumor effects Excellent antimicrobial activity | [89,90] |
| Manganese oxide | High oxygen production potential Overcomes tumor hypoxia Improve anti-tumor effects High light absorption strength absorption ability Excellent biocompatibility | [91] |
| Titanium dioxide | Good photosensitive agent Ability to produce singlet oxygen Allows for bandgap and band position, Highly photostable Non-toxicity, Excellent catalytic activity, Highly abundance and affordability | [89,91] |
6.2. Functionalized Nanomedicine for Targeted PDD and PDT of Esophageal Cancer
Passive and Active Functionalized Nanomedicine for Targeted PDD and PDT of Esophageal Cancer
6.3. Nano-Immunoconjugates for PDD and PDT of Esophageal Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nature Rev. Dis. Prim. 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Then, E.O.; Lopez, M.; Saleem, S.; Gayam, V.; Sunkara, T.; Culliford, A.; Gaduputi, V. Esophageal Cancer: An Updated Surveillance Epidemiology and End Results Database Analysis. World J. Oncol. 2020, 11, 55–64. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Zhu, Y.; Braun, M.; Brüggmann, D.; Schöffel, N.; Groneberg, D.A. A world map of esophagus cancer research: A critical accounting. J. Transl. Med. 2019, 17, 150. [Google Scholar] [CrossRef] [Green Version]
- Spataro, J.; Zfass, A.M.; Schubert, M.; Shah, T. Early Esophageal Cancer: A Gastroenterologist’s Disease. Dig. Dis. Sci. 2019, 64, 3048–3058. [Google Scholar] [CrossRef]
- Thrumurthy, S.G.; Chaudry, M.A.; Thrumurthy, S.S.D.; Mughal, M. Oesophageal cancer: Risks, prevention, and diagnosis. BMJ 2019, 366, l4373. [Google Scholar] [CrossRef]
- Cummings, D.; Wong, J.; Palm, R.; Hoffe, S.; Almhanna, K.; Vignesh, S. Epidemiology, Diagnosis, Staging and Multimodal Therapy of Esophageal and Gastric Tumors. Cancers 2021, 13, 582. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef] [Green Version]
- D’Journo, X.B.; Thomas, P.A. Current management of esophageal cancer. J. Thorac. Dis. 2014, 6, S253–S264. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Moaven, O.; Wang, T.N. Combined Modality Therapy for Management of Esophageal Cancer: Current Approach Based on Experiences from East and West. Surg. Clin. 2019, 99, 479–499. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers; V.4.2021; NCCN: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Ikeda, G.; Yamamoto, S.; Kato, K. The safety of current treatment options for advanced esophageal cancer after first-line chemotherapy. Expert Opin. Drug Saf. 2021, 1–11. [Google Scholar] [CrossRef]
- Shah, M.A.; Bennouna, J.; Doi, T.; Shen, L.; Kato, K.; Adenis, A.; Mamon, H.J.; Moehler, M.; Fu, X.; Cho, B.C.; et al. KEYNOTE-975 study design: A Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Fut. Oncol. 2021, 17, 1143–1153. [Google Scholar] [CrossRef]
- He, S.; Xu, J.; Liu, X.; Zhen, Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Yura, M.; Koyanagi, K.; Hara, A.; Hayashi, K.; Tajima, Y.; Kaneko, Y.; Fujisaki, H.; Hirata, A.; Takano, K.; Hongo, K.; et al. Unresectable esophageal cancer treated with multiple chemotherapies in combination with chemoradiotherapy: A case report. World J. Clin. Cases 2021, 9, 2801–2810. [Google Scholar] [CrossRef]
- Yagi, K.; Toriumi, T.; Aikou, S.; Yamashita, H.; Seto, Y. Salvage treatment after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Ann. Gastroenterol. Surg. 2021, 5, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) forlocal failure afterchemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017, 8, 22135–22144. [Google Scholar] [CrossRef]
- Ishida, N.; Osawa, S.; Miyazu, T.; Kaneko, M.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Furuta, T.; et al. Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience. J. Clin. Med. 2020, 9, 1509. [Google Scholar] [CrossRef]
- Hatogai, K.; Yano, T.; Kojima, T.; Onozawa, M.; Daiko, H.; Nomura, S.; Yoda, Y.; Doi, T.; Kaneko, K.; Ohtsu, A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest. Endosc. 2016, 83, 1130–1139.e1133. [Google Scholar] [CrossRef]
- Koo, M.M.; Unger-Saldaña, K.; Mwaka, A.D.; Corbex, M.; Ginsburg, O.; Walter, F.M.; Calanzani, N.; Moodley, J.; Rubin, G.P.; Lyratzopoulos, G. Conceptual Framework to Guide Early Diagnosis Programs for Symptomatic Cancer as Part of Global Cancer Control. JCO Glob. Oncol. 2021, 35–45. [Google Scholar] [CrossRef]
- Hu, Y.; Masamune, K. Flexible laser endoscope for minimally invasive photodynamic diagnosis (PDD) and therapy (PDT) toward efficient tumor removal. Opt. Express 2017, 25, 16795–16812. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Tanaka, K.; Kishi, K.; Takahashi, H.; Inoue, M.; Saito, T.; Sugimura, K.; Fujiwara, Y.; Ishikawa, O.; et al. Intraoperative photodynamic diagnosis of lymph node metastasis in esophageal cancer patients using 5-aminolevulinic acid. Oncol. Lett. 2015, 10, 3035–3039. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yang, L.; Yi, W.; Fan, W.; Wen, Y.; Miao, X.; Xiong, L. Combination of Fluorescence-Guided Surgery With Photodynamic Therapy for the Treatment of Cancer. Mol. Imaging 2017, 16, 1536012117722911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M.; Tanaka, M.; Ichikawa, H.; Suzuki, T.; Nishie, H.; Ozeki, K.; Shimura, T.; Kubota, E.; Tanida, S.; Kataoka, H. 5-aminolaevulinic acid (5-ALA) accumulates in GIST-T1 cells and photodynamic diagnosis using 5-ALA identifies gastrointestinal stromal tumors (GISTs) in xenograft tumor models. PLoS ONE 2021, 16, e0249650. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I photodynamic therapy by organic–inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, K.P.; Mo, J.G.; Xiong, L.; Wen, Y. Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species. World J. Stem Cells 2020, 12, 562–584. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, P.; de Rooij, F.; Van Velthuysen, M.; Edixhoven, A.; Van Hillegersberg, R.; Tilanus, H.; Wilson, J.; Siersema, P. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: A study in patients with (pre) malignant lesions of the oesophagus. Br. J. Cancer 1998, 78, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denzinger, S.; Burger, M.; Walter, B.; Knuechel, R.; Roessler, W.; Wieland, W.F.; Filbeck, T. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007, 69, 675–679. [Google Scholar] [CrossRef]
- Kishi, K.; Fujiwara, Y.; Yano, M.; Inoue, M.; Miyashiro, I.; Motoori, M.; Shingai, T.; Gotoh, K.; Takahashi, H.; Noura, S. Staging laparoscopy using ALA-mediated photodynamic diagnosis improves the detection of peritoneal metastases in advanced gastric cancer. J. Surg. Oncol. 2012, 106, 294–298. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; Group, A.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Dunn, J.; Lovat, L. Photodynamic therapy using 5-aminolaevulinic acid for the treatment of dysplasia in Barrett’s oesophagus. Expert Opin. Pharmacother. 2008, 9, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.D.; Dunn, J.M.; Selvasekar, C.R.; Mosse, C.A.; Thorpe, S.M.; Novelli, M.R.; Bown, S.G.; Lovat, L.B. Optimal conditions for successful ablation of high-grade dysplasia in Barrett’s oesophagus using aminolaevulinic acid photodynamic therapy. Lasers Med. Sci. 2009, 24, 729–734. [Google Scholar] [CrossRef]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Nishimaki, T.; Tanaka, O.; Ando, N.; Ide, H.; Watanabe, H.; Shinoda, M.; Takiyama, W.; Yamana, H.; Ishida, K.; Isono, K. Evaluation of the accuracy of preoperative staging in thoracic esophageal cancer. Ann. Thorac. Surg. 1999, 68, 2059–2064. [Google Scholar] [CrossRef]
- Wu, L.F.; Wang, B.Z.; Feng, J.L.; Cheng, W.R.; Liu, G.R.; Xu, X.H.; Zheng, Z.C. Preoperative TN staging of esophageal cancer: Comparison of miniprobe ultrasonography, spiral CT and MRI. World J. Gastroenterol. 2003, 9, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Ishihara, R. Photodynamic Therapy for Esophageal Cancer. Clin. Endosc. 2020, 54, 494–498. [Google Scholar] [CrossRef]
- Craig, C.; Gray, J.; Macpherson, M.; Hodgson, H.; Zammit, M.; Fullarton, G. Porfimer sodium photodynamic therapy in the treatment of early oesophageal carcinoma. Photodiagn. Photodyn. Ther. 2007, 4, 244–248. [Google Scholar] [CrossRef]
- Nakamura, T.; Fukui, H.; Shirakawa, K.; Fujii, Y.; Fujimori, T.; Terano, A. Photodynamic therapy of superficial esophageal cancer with a transparent hood. J. Gastrointest. Endosc. 2004, 60, 120–124. [Google Scholar] [CrossRef]
- Tanaka, T.; Matono, S.; Nagano, T.; Murata, K.; Sueyoshi, S.; Yamana, H.; Shirouzu, K.; Fujita, H. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest. Endosc. 2011, 73, 1–6. [Google Scholar] [CrossRef]
- Amanuma, Y.; Horimatsu, T.; Ohashi, S.; Tamaoki, M.; Muto, M. Association of local complete response with prognosis after salvage photodynamic therapy for esophageal squamous cell carcinoma. Dig. Endosc. 2021, 33, 355–363. [Google Scholar] [CrossRef]
- Mehraban, N.; Freeman, H.S. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Nava, H.R.; Allamaneni, S.S.; Dougherty, T.J.; Cooper, M.T.; Tan, W.; Wilding, G.; Henderson, B.W. Photodynamic therapy (PDT) using HPPH for the treatment of precancerous lesions associated with Barrett’s esophagus. Lasers Surg. Med. 2011, 43, 705–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovat, L.B.; Jamieson, N.F.; Novelli, M.R.; Mosse, C.A.; Selvasekar, C.; Mackenzie, G.D.; Thorpe, S.M.; Bown, S.G. Photodynamic therapy with m-tetrahydroxyphenyl chlorin for high-grade dysplasia and early cancer in Barrett’s columnar lined esophagus. Gastrointest. Endosc. 2005, 62, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Asahina, Y.; Nakanishi, H.; Terashima, T.; Okamoto, K.; Yamada, S.; Takatori, H.; Kitamura, K.; Mizukoshi, E.; Ninomiya, I.J.E. Evaluation of the efficacy and safety of salvage photodynamic therapy by talaporfin sodium for cervical esophageal cancers and lesions larger than 3 cm. Esophagus 2020, 18, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Kresfelder, T.L.; Cronjé, M.J.; Abrahamse, H. The effects of two metallophthalocyanines on the viability and proliferation of an esophageal cancer cell line. Photomed. Laser Surg. 2009, 27, 625–631. [Google Scholar] [CrossRef]
- Kuzyniak, W.; Schmidt, J.; Glac, W.; Berkholz, J.; Steinemann, G.; Hoffmann, B.; Ermilov, E.A.; Gürek, A.G.; Ahsen, V.; Nitzsche, B.; et al. Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int. J. Oncol. 2017, 50, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Seotsanyana-Mokhosi, I.; Kresfelder, T.; Abrahamse, H.; Nyokong, T. The effect of Ge, Si and Sn phthalocyanine photosensitizers on cell proliferation and viability of human oesophageal carcinoma cells. J. Photochem. Photobiol. B Biol. 2006, 83, 55–62. [Google Scholar] [CrossRef]
- Firczuk, M.; Winiarska, M.; Szokalska, A.; Jodlowska, M.; Swiech, M.; Bojarczuk, K.; Salwa, P.; Nowis, D. Approaches to improve photodynamic therapy of cancer. Front. Biosci. 2011, 16, 208–224. [Google Scholar] [CrossRef] [Green Version]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Kosaka, H.; Matsui, K.; Ishizaki, M.; Matsushima, H.; Tsuda, T.; Hishikawa, H.; Okumura, T.; Sekimoto, M. Near-Infrared Fluorescence Imaging and Photodynamic Therapy for Liver Tumors. Front. Oncol. 2021, 11, 505. [Google Scholar] [CrossRef]
- Akopov, A.; Rusanov, A.; Gerasin, A.; Kazakov, N.; Urtenova, M.; Chistyakov, I. Preoperarive endobronchial photodinamic therapy improves resectability in initially irresectable (inoperable) locally advanced non small cell lung cancer. Photodiagn. Photodyn. Ther. 2014, 11, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Akimoto, J.; Maruyama, T. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Poorten, V.; Meulemans, J.; Nuyts, S. Postoperative photodynamic therapy as a new adjuvant treatment after robot-assisted salvage surgery of recurrent squamous cell carcinoma of the base of tongue. World J. Surg. Oncol. 2015, 13, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics 2021, 10, 3135–3151. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2020, 33, 7–15. [Google Scholar] [CrossRef]
- Hartmans, E.; Linssen, M.D.; Sikkens, C.; Levens, A.; Witjes, M.J.; van Dam, G.M.; Nagengast, W.B. Tyrosine kinase inhibitor induced growth factor receptor upregulation enhances the efficacy of near-infrared targeted photodynamic therapy in esophageal adenocarcinoma cell lines. Oncotarget 2017, 8, 29846–29856. [Google Scholar] [CrossRef] [Green Version]
- Pye, H.; Butt, M.A.; Funnell, L.; Reinert, H.W.; Puccio, I.; Rehman Khan, S.U.; Saouros, S.; Marklew, J.S.; Stamati, I.; Qurashi, M.; et al. Using antibody directed phototherapy to target oesophageal adenocarcinoma with heterogeneous HER2 expression. Oncotarget 2018, 9, 22945–22959. [Google Scholar] [CrossRef] [Green Version]
- Katsube, R.; Noma, K.; Ohara, T.; Nishiwaki, N.; Kobayashi, T.; Komoto, S.; Sato, H.; Kashima, H.; Kato, T.; Kikuchi, S.; et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021, 11, 1693. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788. [Google Scholar] [CrossRef]
- Singh, B.; Mitragotri, S. Harnessing cells to deliver nanoparticle drugs to treat cancer. Biotechnol. Adv. 2020, 42, 107339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Chen, H.; Gao, Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur. J. Pharm. Sci. 2020, 144, 105213. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic nanoparticle based active targeting for photodynamic therapy treatment of breast cancer cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Kruger, C.A.; Abrahamse, H. Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Molecules 2018, 23, 2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.A.; Azzazy, H.M.E.; Schaefer, J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Maurya, S.D.; Das, M.K.; Tilak, V.K.; Verma, K.K.; Dhakar, R.C. Dendrimers in drug delivery, diagnosis and therapy: Basics and potential applications. J. Drug Deliv. Ther. 2016, 6, 67–92. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Nanocarriers in photodynamic therapy-in vitro and in vivo studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1509. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Lu, D.; Tao, R.; Wang, Z. Carbon-based materials for photodynamic therapy: A mini-review. Front. Chem. Sci. Eng. 2019, 13, 310–323. [Google Scholar] [CrossRef]
- Xue, M.; Zhao, J.; Zhan, Z.; Zhao, S.; Lan, C.; Ye, F.; Liang, H. Dual functionalized natural biomass carbon dots from lychee exocarp for cancer cell targetable near-infrared fluorescence imaging and photodynamic therapy. Nanoscale 2018, 10, 18124–18130. [Google Scholar] [CrossRef]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- El-Hussein, A. Study DNA damage after photodynamic therapy using silver nanoparticles with A549 cell line. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar]
- Yan, K.; Zhang, Y.; Mu, C.; Xu, Q.; Jing, X.; Wang, D.; Dang, D.; Meng, L.; Ma, J. Versatile Nanoplatforms with enhanced Photodynamic Therapy: Designs and Applications. Theranostics 2020, 10, 7287–7318. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Bara’nska, E.; Wieche´c-Cudak, O.; Rak, M.; Bienia, A.; Mrozek-Wilczkiewicz, A.; Krzykawska-Serda, M.; Serda, M. Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model. Nanomaterials 2021, 11, 513. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anticancer Photodynamic Therapy Properties of Sulfur-Doped Graphene Quantum Dot and Methylene Blue Preparations in MCF-7 Breast Cancer Cell Culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef]
- Fernandes, S.R.G.; Fernandes, R.; Sarmento, B.; Pereira, P.M.R.; Tomé, J.P.C. Photoimmunoconjugates: Novel synthetic strategies to target and treat cancer by photodynamic therapy. Org. Biomol. Chem. 2019, 17, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Tsung, A.; Hu, Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules 2020, 25, 4964. [Google Scholar] [CrossRef]
- Li, Q.; Gu, W.; Liu, K.; Xiao, N.; Zhang, J.; Shao, L.; Li, L.; Zhang, S.; Li, P. RGD conjugated, Cy5.5 labeled polyamidoamine dendrimers for targeted near-infrared fluorescence imaging of esophageal squamous cell carcinoma. RSC Adv. 2016, 6, 74560–74566. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Qiu, S.; Yu, J. Facile fabrication of Cu9-S5 loaded core-shell nanoparticles for near infrared radiation mediated tumor therapeutic strategy in human esophageal squamous carcinoma cells nursing care of esophageal cancer patients. J. Photochem. Photobiol. B Biol. 2019, 199, 111583. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Kang, L.; Li, C.; Xu, Z.; Li, J.; Zhang, M. Fluoroscopy-Guided Salvage Photodynamic Therapy Combined with Nanoparticle Albumin-Bound Paclitaxel for Locally Advanced Esophageal Cancer after Chemoradiotherapy: A Case Report and Literature Review. Cancer Biotherapy Radiopharm. 2021. [Google Scholar] [CrossRef]
- Ji, C.; Ju, S.; Zhang, D.; Qiang, J. Nanomedicine Based N-Trimethyl Chitosan Entangled Solid Lipid Nanoparticle Loaded with Irinotecan to Enhance the Therapeutic Efficacy in Esophageal Cancer Cells. J. Biomater. Tissue Eng. 2018, 8, 1195–1200. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Zhang, A.; Guo, Y.; Fan, S.; He, Y.; Yang, K.; Wang, J.; Cui, D.; Cheng, Y. Carbon nanocage-based nanozyme as an endogenous H2O2-activated oxygenerator for real-time bimodal imaging and enhanced phototherapy of esophageal cancer. Nanoscale 2020, 12, 21674–21686. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chang, Y.; Cui, C.; Sun, L.; Wang, D.H.; Pan, Z.; Zhang, M. Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nat. Commun. 2018, 9, 2605. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Kang, W.; Li, N.; Guo, F.; Chang, H.; Wei, W. Multifunctional gold nanoparticle based selective detection of esophageal squamous cell carcinoma cells using resonance Rayleigh scattering assay. Microchem. J. 2021, 163, 105905. [Google Scholar] [CrossRef]
- Wang, Y.W.; Kang, S.; Khan, A.; Bao, P.Q.; Liu, J.T.C. In vivo multiplexed Mol. Imaging of esophageal cancer via spectral endoscopy of topically applied SERS nanoparticles. Biomed. Opt. Express 2015, 6, 3714–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Organic NP Platforms | Characteristics | Reference |
|---|---|---|
| Liposome | They are bilayer phospholipid systems High biocompatibility Nano drug carrier for both soluble and insoluble drugs Limits dark toxicity associated with conventional PSs Enhance cellular uptake | [73,77,82] |
| Micelle | Amphiphilic self-assembled structure Exist in different nanoforms Facilitate delivery of hydrophobic and hydrophilic PS drug Enhance both passive and active drug delivery Easy surface functionalization High stability in suspension and biocompatibility | [73,77] |
| Dendrimer | The monodisperse and highly branched structure Sustained drug release, High solubilization potential, Increase drug payload Enhance colloidal, biocompatibility, and shelf stability | [77,83] |
| Polymeric NPs | Easy to formulate and synthesize Exit in diverse structure Good biocompatibility Increase drug permeability into the target site Allow for surface modification Increase drug solubility Protection drugs from biodegradation An excellent delivery system for PS | [84] |
| Carbon-Based NP Platforms | Characteristics | References |
|---|---|---|
| Carbon nanotube | High photothermal conversion strength Tunable fluorescence Allow for surface engineering Efficient nanocarrier for insoluble PS drugs Suitable candidate for PDT cancer treatment Allows for covalent and non-covalent modification attachment of PS drug Enhance photocytotoxic effect | [86,92] |
| Fullerenes | Allows for extensive functionalization, Photochemistry potentials Ability to self-assemble into supramolecular fullerosomes Resistance to photobleaching Serve as drug nanocarrier to the nuclear pore complex and tumor vasculature | [93,94] |
| Graphene | Good photothermal property High singlet oxygen production Allow high drug payload Increase generation of ROS High Electrical thermal capacity Enhance cellular internalization Improve anti-tumor efficiency of PDT | [88,92,95] |
| Nanoparticle Platform | PS/Drug | Ligand | Target | Outcome | Ref. |
|---|---|---|---|---|---|
| Dendrimer | NIR dye Cy5.5 | Cyclic RGDfK peptide | αvβ3 integrin | Facilitate early detection of esophageal cancer | [99] |
| Core-shell silica materials (Cu9S5@MS) | NIR copper-based chalcogenide | - | - | Effective nanocarrier Excellent cancer cell death | [100] |
| Albumin | Paclitaxel | - | - | Enhance tumor elimination Organ preservation Excellent for locally advanced esophageal cancer | [101] |
| Solid lipid nanoparticle | Chitosan coated irinotecan | Increase drug internalization Promote tumor destruction | [102] | ||
| Manganese oxide/Carbon nanocages | IR800 | Albumin | - | Increase drug payload Increase drug release and cellular uptake Increase ROS production Allows for visualization | [103] |
| Fluorescent-peptide nanoparticles | Epirubicin | RGD | αvβ3 integrin | Enhance drug release and internalization Promote tumor visualization | [104] |
| Gold nanoparticles | Antibody/aptamer | HER2/EGFR | Improve and facilitate rapid esophageal cancer detection Very sensitive technique | [105,106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didamson, O.C.; Abrahamse, H. Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine. Pharmaceutics 2021, 13, 1943. https://doi.org/10.3390/pharmaceutics13111943
Didamson OC, Abrahamse H. Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine. Pharmaceutics. 2021; 13(11):1943. https://doi.org/10.3390/pharmaceutics13111943
Chicago/Turabian StyleDidamson, Onyisi Christiana, and Heidi Abrahamse. 2021. "Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine" Pharmaceutics 13, no. 11: 1943. https://doi.org/10.3390/pharmaceutics13111943
APA StyleDidamson, O. C., & Abrahamse, H. (2021). Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine. Pharmaceutics, 13(11), 1943. https://doi.org/10.3390/pharmaceutics13111943