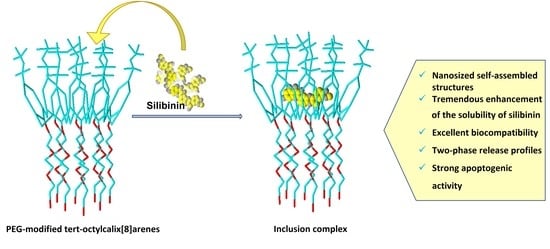
PEG-Modified tert-Octylcalix[8]arenes as Drug Delivery Nanocarriers of Silibinin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amphiphilic PEGylated tert-Octylcalix[8]arenes
2.2.1. 1H NMR and DOSY Characterization
2.2.2. Determination of the Critical Micellization Concentration (CMC)
2.3. Preparation of Inclusion Complexes of Silibinin and PEGylated tert-Octylcalix[8]arenes
Solvent Evaporation Method
2.4. Characterization of SBN:PEGylated tert-Octylcalix[8]arenes Inclusion Complexes and Supramolecular Aggregates
2.4.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.2. Dynamic Light Scattering (DLS)
2.4.3. Electrophoretic Light Scattering
2.5. In Vitro Release Study
2.6. Cytotoxicity Evaluation
2.6.1. Cell lines and Cultured Conditions
2.6.2. MTT Dye Reduction Assay
3. Results and Discussion
3.1. Synthesis of Amphiphilic tert-Octylcalix[8]arenes
3.2. Aqueous Solution Properties
3.3. Phase Solubility Evaluation
3.4. Characterization of OEC:SBN Inclusion Complexes
Fourier Transform Infrared (FT-IR) Spectroscopy
3.5. Characterization of Silibinin-Loaded OEC Supramolecular Aggregates
3.5.1. Size, Size Distribution and Zeta Potential
3.5.2. Silibinin Release Study
3.5.3. Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Salmi, H.A.; Sarna, S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. Scand. J. Gastroenterol. 1982, 17, 517–521. [Google Scholar] [CrossRef]
- Weselowska, O.; Łania-Pietrzak, B.; Kuzdzal, M.; Stanczak, K.; Mosiadz, D.; Dobryszycki, P.; Ozyhar, A.; Komorowska, M.; Hendrich, A.B.; Michalak, K. Influence of silybin on biophysical properties of phospholipid bilayers. Acta Pharmacol. Sin. 2007, 28, 296–306. [Google Scholar] [CrossRef]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and Silymarin—New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk Thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J. Vet. Intern. Med. 2012, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. Photobiol. B Biol. 2021, 216, 112147. [Google Scholar] [CrossRef] [PubMed]
- Vue, B.; Zhang, S.; Zhang, X.; Parisis, K.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. Silibinin derivatives as anti-prostate cancer agents: Synthesis and cell-based evaluations. Eur. J. Med. Chem. 2016, 109, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahira, S.; Kommineni, N.; Husain, G.M.; Khan, W. Cabazitaxel and Silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomed. Pharmacother. 2019, 110, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Binienda, A.; Ziolkowska, S.; Pluciennik, E. The anticancer properties of Silibinin: Its molecular mechanism and therapeutic effect in breast cancer. Anti-Cancer Agents Med. Chem. 2020, 20, 1787–1796. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R. The use of Silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [Green Version]
- Sahibzada, M.U.; Sadiq, A.; Zahoor, M.; Naz, S.; Shahid, M.; Qureshi, N.A. Enhancement of bioavailability and hepatoprotection by Silibinin through conversion to nanoparticles prepared by Liquid Antisolvent method. Arab. J. Chem. 2020, 13, 3682–3689. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Voinovich, D.; Perissutti, B.; Grassi, M.; Passerini, N.; Bigotto, A. Solid state mechanochemical activation of silybum marianum dry extract with Betacyclodextrins: Characterization and bioavailability of the COGROUND systems. J. Pharm. Sci. 2009, 98, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Oil based nanocarrier for improved oral delivery of Silymarin: In vitro and in vivo studies. Int. J. Pharm. 2011, 413, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Q.; Fuentes-Chandía, M.; Tharmalingam, V.; Ur Rehman, M.A.; Leal-Egaña, A.; Boccaccini, A.R. Silibinin releasing mesoporous bioactive glass nanoparticles with potential for breast cancer therapy. Ceram. Int. 2020, 46, 29111–29119. [Google Scholar] [CrossRef]
- Amirsaadat, S.; Jafari-Gharabaghlou, D.; Alijani, S.; Mousazadeh, H.; Dadashpour, M.; Zarghami, N. Metformin and Silibinin co-loaded PLGA-peg nanoparticles for effective combination therapy against human breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102107. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; Zayed, R.; Shukr, M.H.; Elsayed, I. Sucrose acetate isobutyrate based nanovesicles: A promising platform for drug delivery and bioavailability enhancement. J. Drug Deliv. Sci. Technol. 2020, 58, 101806. [Google Scholar] [CrossRef]
- Yazdi Rouholamini, S.E.; Moghassemi, S.; Maharat, Z.; Hakamivala, A.; Kashanian, S.; Omidfar, K. Effect of silibinin-loaded nano-NIOSOMAL coated with trimethyl chitosan on mirnas expression in 2D and 3D models of T47D Breast Cancer Cell Line. Artif. Cells Nanomed. Biotechnol. 2017, 46, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, M.; Abolmaali, S.S.; Tamaddon, A.M.; Abedanzadeh, M.; Abedi, M. One-pot synthesis of poly(alkyl methacrylate)-functionalized mesoporous silica hybrid nanocomposites for microencapsulation of poorly soluble phytochemicals. Colloid Interface Sci. Commun. 2020, 37, 100298. [Google Scholar] [CrossRef]
- Gohulkumar, M.; Gurushankar, K.; Rajendra Prasad, N.; Krishnakumar, N. Enhanced cytotoxicity and apoptosis-induced anticancer effect of Silibinin-loaded nanoparticles in oral carcinoma (KB) cells. Mater. Sci. Eng. C 2014, 41, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, X. Development of calixarene-based drug nanocarriers. J. Mol. Liq. 2021, 325, 115246. [Google Scholar] [CrossRef]
- Drakalska, E.; Momekova, D.; Manolova, Y.; Budurova, D.; Momekov, G.; Genova, M.; Antonov, L.; Lambov, N.; Rangelov, S. Hybrid liposomal pegylated calix[4]arene systems as drug delivery platforms for curcumin. Int. J. Pharm. 2014, 472, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostos, F.J.; Lebrón, J.A.; López-Cornejo, P.; López-López, M.; García-Calderón, M.; García-Calderón, C.B.; Rosado, I.V.; Kalchenko, V.I.; Rodik, R.V.; Moyá, M.L. Self-aggregation in aqueous solution of amphiphilic cationic calix[4]arenes. potential use as vectors and nanocarriers. J. Mol. Liq. 2020, 304, 112724. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Syakaev, V.V.; Shalaeva, Y.V.; Sapunova, A.S.; Voloshina, A.D.; Gubaidullin, A.T.; Bazanova, O.B.; Babaev, V.M.; Nizameev, I.R.; et al. The PH-responsive calix[4]resorcinarene-MPEG conjugates bearing acylhydrazone bonds: Synthesis and study of the potential as supramolecular drug delivery systems. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124453. [Google Scholar] [CrossRef]
- Casnati, A.; Sansone, F.; Ungaro, R. Peptido- and glycocalixarenes: playing with hydrogen bonds around hydrophobic cavities. Acc. Chem. Res. 2003, 36, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.; Lazar, A.N.; Coleman, A.W. Biopharmaceutical applications of Calixarenes. J. Drug Deliv. Sci. Technol. 2004, 14, 3–20. [Google Scholar] [CrossRef]
- Kunsági-Máté, S.; Szabó, K.; Lemli, B.; Bitter, I.; Nagy, G.; Kollár, L. Host–guest interaction between water-soluble calix[6]arene hexasulfonate and p-nitrophenol. Thermochim. Acta 2005, 425, 121–126. [Google Scholar] [CrossRef]
- Perret, F.; Lazar, A.N.; Coleman, A.W. Biochemistry of the para-sulfonato-calix[n]arenes. Chem. Commun. 2006, 23, 2425–2438. [Google Scholar] [CrossRef]
- Martin, A.D.; Raston, C.L. Multifunctional P-phosphonated calixarenes. Chem. Commun. 2011, 47, 9764. [Google Scholar] [CrossRef]
- Fulton, D.A.; Stoddart, J.F. Neoglycoconjugates based on cyclodextrins and Calixarenes. Bioconjugate Chem. 2001, 12, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Kuldová, M.; Hulíková, K.; Stibor, I.; Lhoták, P.; Dudič, M.; Budka, J.; Pelantová, H.; Bezouška, K.; Fišerová, A.; et al. Retracted: N-acetyl-D-glucosamine substituted calix[4]arenes as stimulators of NK cell-mediated antitumor immune response. Carbohydr. Res. 2007, 342, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Shahgaldian, P.; Sciotti, M.A.; Pieles, U. Amino-substituted Amphiphilic Calixarenes: Self-assembly and interactions with DNA. Langmuir 2008, 24, 8522–8526. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Sun, M.; Li, H.; Guo, C.; Cui, J.; Li, A.; Cao, F.; Xi, Y.; Lou, H.; et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev. Ind. Pharm. 2010, 36, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Taton, D.; Saule, M.; Logan, J.; Duran, R.; Hou, S.; Chaikof, E.L.; Gnanou, Y. Polymerization of ethylene oxide with a Calixarene-based precursor: Synthesis of eight-arm poly(ethylene oxide) stars by the core-first methodology. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1669–1676. [Google Scholar] [CrossRef]
- Mustafina, A.; Zakharova, L.; Elistratova, J.; Kudryashova, J.; Soloveva, S.; Garusov, A.; Antipin, I.; Konovalov, A. Solution behavior of mixed systems based on novel amphiphilic cyclophanes and Triton X100: Aggregation, Cloud Point Phenomenon and cloud point extraction of lanthanide ions. J. Colloid Interface Sci. 2010, 346, 405–413. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Konstantinov, S.M.; Eibl, H.; Berger, M.R. BCR-abl influences the antileukaemic efficacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–374. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) Triblock copolymers in aqueous solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; London, E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 1984, 139, 408–412. [Google Scholar] [CrossRef]
- Halacheva, S.; Rangelov, S.; Tsvetanov, C. Poly(glycidol)-based analogues to pluronic block copolymers. synthesis and aqueous solution properties. Macromolecules 2006, 39, 6845–6852. [Google Scholar] [CrossRef]
- Scherlund, M.; Brodin, A.; Malmsten, M. Micellization and gelation in block copolymer systems containing local anesthetics. Int. J. Pharm. 2000, 211, 37–49. [Google Scholar] [CrossRef]
- Svensson, M.; Linse, P.; Tjerneld, F. Phase behavior in aqueous two-phase systems containing micelle-forming block copolymers. Macromolecules 1995, 28, 3597–3603. [Google Scholar] [CrossRef]
- Johnson, C.S. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Momekova, D.; Budurova, D.; Drakalska, E.; Shenkov, S.; Momekov, G.; Trzebicka, B.; Lambov, N.; Tashev, E.; Rangelov, S. Aggregation behavior and in vitro biocompatibility study of octopus-shaped macromolecules based on tert-butylcalix[4]arenes. Int. J. Pharm. 2012, 436, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. Correlation and prediction of solvent effects on paper chromatographic RF values. Anal. Chem. 1965, 37, 261–264. [Google Scholar] [CrossRef]
- Ukhatskaya, E.V.; Kurkov, S.V.; Matthews, S.E.; El Fagui, A.; Amiel, C.; Dalmas, F.; Loftsson, T. Evaluation of a cationic calix[4]arene: Solubilization and self-aggregation ability. Int. J. Pharm. 2010, 402, 10–19. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Li, Y.; Xu, K.; Li, S.; Tang, P.; Li, H. The influence of hydroxypropyl-β-cyclodextrin on the solubility, dissolution, cytotoxicity, and binding of riluzole with human serum albumin. J. Pharm. Biomed. Anal. 2016, 117, 453–463. [Google Scholar] [CrossRef]
- Suvarna, V.; Kajwe, A.; Murahari, M.; Pujar, G.V.; Inturi, B.K.; Sherje, A.P. Inclusion complexes of Nateglinide with HP–β–CD and L-arginine for solubility and dissolution enhancement: Preparation, characterization, and Molecular Docking Study. J. Pharm. Innov. 2017, 12, 168–181. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Wang, L.; Wang, L.; Li, Y.; Liu, Y.; Wu, M.; Zhao, X.; Zhang, X. Preparation, characterization and antitumor activity evaluation of Silibinin nanoparticles for oral delivery through Liquid Antisolvent precipitation. RSC Adv. 2017, 7, 54379–54390. [Google Scholar] [CrossRef] [Green Version]
- Furer, V.L.; Vandyukov, A.E.; Zaripov, S.R.; Solovieva, S.E.; Antipin, I.S.; Kovalenko, V.I. FT-IR and FT-raman study of hydrogen bonding in P-alkylcalix[8]arenes. Vib. Spectrosc. 2018, 95, 38–43. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Abbreviation | DP of PEG Chains | Mna | |
|---|---|---|---|
| Theoretical | Experimental a | ||
| OEC- I | 5 | 4 | 3200 |
| OEC-II | 7 | 6 | 3900 |
| OEC- III | 19 | 14 | 6700 |
| OEC-IV | 22 | 17 | 7800 |
| OEC- V | 42 | 41 | 16,200 |
| OEC- VI | 57 | 52 | 20,000 |
| OEC- VII | 100 | 96 | 36,000 |
| Parameter | Slope | R2 | Ks (mL/μmol) | ΔG (kJ/mol) | So (μmol/mL) | δ (%) | |
|---|---|---|---|---|---|---|---|
| Complex | |||||||
| SBN:OEC-IV | 0.73556 | 0.998 | 126.4 | −11.98 | 0.022 | 1877 | |
| SBN:OEC-V | 0.73301 | 0.996 | 124.5 | −11.84 | 1786 | ||
| Sample | Diameter (nm) | PDI | ζ Potential (mV) |
|---|---|---|---|
| OEC-IV empty | 260.0 ± 5.2 | 0.54 | −32.2 ± 1.55 |
| OEC-IV:SBN | 211.0 ± 2.4 | 0.44 | −23.1 ± 0.35 |
| OEC-V empty | 295.0 ± 3.8 | 0,48 | −31.5 ± 0.5 |
| OEC-V:SBN | 200.0 ± 5.6 | 0.39 | −19.9 ± 1.9 |
| Sample | IC50 | |
|---|---|---|
| HL-60 | CAL-29 | |
| SBN | 3.01 | 3.61 |
| OEC-IV:SBN | 4.48 | 4.13 |
| OEC-V:SBN | 4.67 | 4.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budurova, D.; Momekova, D.; Momekov, G.; Shestakova, P.; Penchev, H.; Rangelov, S. PEG-Modified tert-Octylcalix[8]arenes as Drug Delivery Nanocarriers of Silibinin. Pharmaceutics 2021, 13, 2025. https://doi.org/10.3390/pharmaceutics13122025
Budurova D, Momekova D, Momekov G, Shestakova P, Penchev H, Rangelov S. PEG-Modified tert-Octylcalix[8]arenes as Drug Delivery Nanocarriers of Silibinin. Pharmaceutics. 2021; 13(12):2025. https://doi.org/10.3390/pharmaceutics13122025
Chicago/Turabian StyleBudurova, Desislava, Denitsa Momekova, Georgi Momekov, Pavletta Shestakova, Hristo Penchev, and Stanislav Rangelov. 2021. "PEG-Modified tert-Octylcalix[8]arenes as Drug Delivery Nanocarriers of Silibinin" Pharmaceutics 13, no. 12: 2025. https://doi.org/10.3390/pharmaceutics13122025
APA StyleBudurova, D., Momekova, D., Momekov, G., Shestakova, P., Penchev, H., & Rangelov, S. (2021). PEG-Modified tert-Octylcalix[8]arenes as Drug Delivery Nanocarriers of Silibinin. Pharmaceutics, 13(12), 2025. https://doi.org/10.3390/pharmaceutics13122025