Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Macroencapsulation Device and Contents
2.1.1. Device Fabrication
2.1.2. Hyaluronic Acid Gel Formulation (VEGF Diluent)
2.1.3. VEGF Microsphere Formulation
2.2. Sub-Muscular Implantation in Rats
2.3. Tissue Processing
2.4. Blood Vessel Analysis
2.5. Fibrous Capsule Analysis
2.6. Macrophage Response
2.7. Statistical Analysis
3. Results
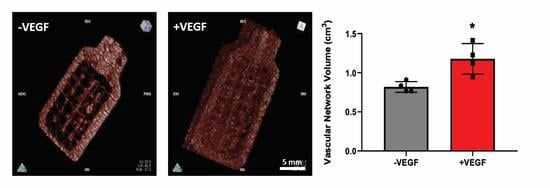
3.1. In Vivo Implantation of VEGF Microspheres within a Macroencapsulation Device Increases Neovascularization
3.2. VEGF Microspheres Increase Vessel Maturity, Stability and Vessel Diameter
3.3. VEGF Microspheres Do Not Cause a Heightened FBR
3.4. VEGF Does Not Cause a Heightened Macrophage Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, N.; Steil, G.M.; Colton, C.K.; Bonner-Weir, S.; Weir, G.C. Improved Vascularization of Planar Membrane Diffusion Devices following Continuous Infusion of Vascular Endothelial Growth Factor. Cell Transplant. 2000, 9, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Homo-Delarche, F.; Boitard, C. Autoimmune diabetes: The role of the islets of langerhans. Immunol. Today 1996, 17, 456–460. [Google Scholar] [CrossRef]
- Ballian, N.; Brunicardi, F.C. Islet Vasculature as a Regulator of Endocrine Pancreas Function. World J. Surg. 2007, 31, 705–714. [Google Scholar] [CrossRef]
- Dionne, K.E.; Colton, C.K.; Lyarmush, M. Effect of Hypoxia on Insulin Secretion by Isolated Rat and Canine Islets of Langerhans. Diabetes 1993, 42, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Harlan, D.M.; Kenyon, N.S.; Korsgren, O.; Roep, B.O.; Immunology of Diabetes Society. Current Advances and Travails in Islet Transplantation. Diabetes 2009, 58, 2175–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedraza, E.; Coronel, M.; Fraker, C.; Ricordi, C.; Stabler, C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA 2012, 109, 4245–4250. [Google Scholar] [CrossRef] [Green Version]
- Pepper, A.R.; Gala-Lopez, B.; Ziff, O.; Shapiro, A.M.J. Revascularization of Transplanted Pancreatic Islets and Role of the Transplantation Site. Clin. Dev. Immunol. 2013, 2013, 352315. [Google Scholar] [CrossRef] [Green Version]
- Smink, A.M.; Hertsig, D.T.; Schwab, L.; van Apeldoorn, A.A.; de Koning, E.; Faas, M.M.; de Haan, B.J.; de Vos, P. A Retrievable, Efficacious Polymeric Scaffold for Subcutaneous Transplantation of Rat Pancreatic Islets. Ann. Surg. 2017, 266, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Smink, A.M.; Li, S.; Hertsig, D.T.; de Haan, B.J.; Schwab, L.; van Apeldoorn, A.; de Koning, E.; Faas, M.M.; Lakey, J.R.; de Vos, P. The Efficacy of a Prevascularized, Retrievable Poly(D,L,-lactide-co-ε-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 2017, 101, e112–e119. [Google Scholar] [CrossRef]
- Bowers, D.T.; Song, W.; Wang, L.-H.; Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 2019, 95, 131–151. [Google Scholar] [CrossRef]
- Pepper, A.R.; Pawlick, R.; Bruni, A.; Gala-Lopez, B.; Wink, J.; Rafiei, Y.; Bral, M.; Abualhassan, N.; Shapiro, A.M.J. Harnessing the Foreign Body Reaction in Marginal Mass Device-less Subcutaneous Islet Transplantation in Mice. Transplantation 2016, 100, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M.J. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015, 33, 518–523. [Google Scholar] [CrossRef]
- Padera, R.F.; Colton, C.K. Time course of membrane microarchitecture-driven neovascularization. Biomaterials 1996, 17, 277–284. [Google Scholar] [CrossRef]
- Colton, C.K. Oxygen supply to encapsulated therapeutic cells. Adv. Drug Deliv. Rev. 2014, 67–68, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Brauker, J.H.; Carr-Brendel, V.E.; Martinson, L.A.; Crudele, J.; Johnston, W.D.; Johnson, R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995, 29, 1517–1524. [Google Scholar] [CrossRef]
- Auger, F.A.; Gibot, L.; Lacroix, D. The Pivotal Role of Vascularization in Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Coulter, F.B.; Levey, R.E.; Robinson, S.T.; Dolan, E.B.; Deotti, S.; Monaghan, M.; Dockery, P.; Coulter, B.S.; Burke, L.P.; Lowery, A.J.; et al. Additive Manufacturing of Multi-Scale Porous Soft Tissue Implants That Encourage Vascularization and Tissue Ingrowth. Adv. Healthc. Mater. 2021, 10, 2100229. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Aquart, J.; Johnson, C.T.; Shea, L.D.; Shirwan, H.; García, A.J. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017, 3, e1700184. [Google Scholar] [CrossRef] [Green Version]
- Vallbacka, J.J.; Sefton, M.V. Vascularization and Improved In Vivo Survival of VEGF-Secreting Cells Microencapsulated in HEMA-MMA. Tissue Eng. 2007, 13, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Alexander, M.; Robles, L.; Foster, C.E.; Lakey, J.R. Islet and Stem Cell Encapsulation for Clinical Transplantation. Rev. Diabet. Stud. 2014, 11, 84–101. [Google Scholar] [CrossRef] [Green Version]
- Tilakaratne, H.; Hunter, S.K.; Andracki, M.E.; Benda, J.A.; Rodgers, V. Characterizing short-term release and neovascularization potential of multi-protein growth supplement delivered via alginate hollow fiber devices. Biomaterials 2007, 28, 89–98. [Google Scholar] [CrossRef]
- Balamurugan, A.N.; Gu, Y.; Tabata, Y.; Miyamoto, M.; Cui, W.; Hori, H.; Satake, A.; Nagata, N.; Wang, W.; Inoue, K. Bioartificial Pancreas Transplantation at Prevascularized Intermuscular Space: Effect of Angiogenesis Induction on Islet Survival. Pancreas 2003, 26, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, A.; Molano, R.D.; Ricordi, C.; Zahr, E.; Collins, J.; Valdes, R.; Inverardi, L. Reversal of Diabetes by Pancreatic Islet Transplantation into a Subcutaneous, Neovascularized Device. Transplantation 2006, 81, 1318–1324. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Satake, A.; Sumi, S.; Inoue, K.; Nagata, N.; Tabata, Y.; Miyakoshi, J. The Efficient Prevascularization Induced by Fibroblast Growth Factor 2 With a Collagen-Coated Device Improves the Cell Survival of a Bioartificial Pancreas. Pancreas 2004, 28, e70–e79. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, A.J.; Davies, N.; Oosthuysen, A.; Chokoza, C.; Zilla, P.; Bezuidenhout, D. Improved vascularization of porous scaffolds through growth factor delivery from heparinized polyethylene glycol hydrogels. Acta Biomater. 2017, 49, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.; Manzoli, V.; Abreu, M.; Villa, C.; Martino, M.M.; Molano, R.D.; Torrente, Y.; Pileggi, A.; Inverardi, L.; Ricordi, C.; et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol. Bioeng. 2015, 112, 1916–1926. [Google Scholar] [CrossRef]
- Phelps, E.; Templeman, K.L.; Thulé, P.M.; García, A.J. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 2013, 5, 125–136. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.; Cryan, S. Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, K.C.; Coulter, F.; Maas-Bakker, R.F.; Ghersi, G.; Nguyen, T.T.; Steendam, R.; Duffy, G.P.; Hennink, W.E.; O’Cearbhaill, E.; Kok, R.J. Vascular Endothelial Growth Factor–Releasing Microspheres Based on Poly(ε-Caprolactone-PEG-ε-Caprolactone)-b-Poly(L-Lactide) Multiblock Copolymers Incorporated in a Three-Dimensional Printed Poly(Dimethylsiloxane) Cell Macroencapsulation Device. J. Pharm. Sci. 2020, 109, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, K.C.; Maas-Bakker, R.F.; Nguyen, T.T.; Duarte, A.M.; Hendriks, G.; Sequeira, L.; Duffy, G.P.; Steendam, R.; Hennink, W.E.; Kok, R.J. Sustained Release of Vascular Endothelial Growth Factor from Poly(ε-caprolactone-PEG-ε-caprolactone)-b-Poly(l-lactide) Multiblock Copolymer Microspheres. ACS Omega 2019, 4, 11481–11492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, B.; Robinson, S.; Holcombe, S.; Levey, R.; Dockery, P.; Johnson, P.; Wang, S.; Dolan, E. Duffy, Developing a morphomics framework to optimise implant site-specific design parameters for islet macroencapsulation devices. J. R. Soc. Interface 2021. [Google Scholar] [CrossRef]
- Davies, N.; Dobner, S.; Bezuidenhout, D.; Schmidt, C.; Beck, M.; Zisch, A.H.; Zilla, P. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials 2008, 29, 3531–3538. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, E.B.; Varela, C.E.; Mendez, K.; Whyte, W.; Levey, R.E.; Robinson, S.T.; Maye, E.; O’Dwyer, J.; Beatty, R.; Rothman, A.; et al. An actuatable soft reservoir modulates host foreign body response. Sci. Robot. 2019, 4, eaax7043. [Google Scholar] [CrossRef] [Green Version]
- Howard, C.; Reed, M. Unbiased Stereology, 2nd ed.; QTP Publications: Coleraine, UK, 2010. [Google Scholar]
- Dockery, P.; Fraher, J. The quantification of vascular beds: A stereological approach. Exp. Mol. Pathol. 2007, 82, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G.; Jensen, E.B. Stereological estimation of the volume-weighted mean volume of arbitrary particles observed on random sections. J. Microsc. 1985, 138, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.V.; Reed, M. Unbiased Stereology: Three-Dimensional Measurement in Microscopy, 1st ed.; Garland Science/BIOS Scientific Publisher: Oxford, UK, 1998. [Google Scholar]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, W.; Stratman, A.N.; Sacharidou, A.; Davis, G.E. Chapter 5 In Vitro Three Dimensional Collagen Matrix Models of Endothelial Lumen Formation During Vasculogenesis and Angiogenesis. In Methods in Enzymology; Elsevier B.V.: Amsterdam, The Netherlands, 2008; Volume 443, pp. 83–101. [Google Scholar]
- Monaghan, M.G.; Holeiter, M.; Brauchle, E.; Layland, S.L.; Lu, Y.; Deb, A.; Pandit, A.; Nsair, A.; Schenke-Layland, K. Exogenous miR-29B Delivery Through a Hyaluronan-Based Injectable System Yields Functional Maintenance of the Infarcted Myocardium. Tissue Eng. Part A 2018, 24, 57–67. [Google Scholar] [CrossRef]
- Dolan, E.B.; Hofmann, B.; de Vaal, M.H.; Bellavia, G.; Straino, S.; Kovarova, L.; Pravda, M.; Velebny, V.; Daro, D.; Braun, N.; et al. A bioresorbable biomaterial carrier and passive stabilization device to improve heart function post-myocardial infarction. Mater. Sci. Eng. C 2019, 103, 109751. [Google Scholar] [CrossRef]
- Bell, M.A.; Ball, M.J. Morphometric comparison of hippocampal microvasculature in ageing and demented people: Diameters and densities. Acta Neuropathol. 1981, 53, 299–318. [Google Scholar] [CrossRef]
- Bennett, R.E.; Robbins, A.B.; Hu, M.; Cao, X.; Betensky, R.A.; Clark, T.; Das, S.; Hyman, B.T. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1289–E1298. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.Y.; Tozzi, C.A.; Babiarz, J.; Leppert, P.C. Collagen Changes in Rat Cervix in Pregnancy—Polarized Light Microscopic and Electron Microscopic Studies. Proc. Soc. Exp. Biol. Med. 1995, 209, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Wolman, M.; Kasten, F.H. Polarized light microscopy in the study of the molecular structure of collagen and reticulin. Histochem. Cell Biol. 1986, 85, 41–49. [Google Scholar] [CrossRef]
- Szendröi, M.; Vajta, G.; Kovács, L.; Schaff, Z.; Lapis, K. Polarization colours of collagen fibres: A sign of collagen production activity in fibrotic processes. Acta Morphol. Hung. 1984, 32, 47–55. [Google Scholar] [PubMed]
- Conway, E.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.J.; Little, C.D. VEGF and Vascular Fusion: Implications for Normal and Pathological Vessels. J. Histochem. Cytochem. 1999, 47, 1351–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, M.; Carrer, A.; Moimas, S.; Zandonà, L.; Bussani, R.; Casagranda, B.; Palmisano, S.; Prelazzi, P.; Giacca, M.; Zentilin, L.; et al. VEGF121 and VEGF165 differentially promote vessel maturation and tumor growth in mice and humans. Cancer Gene Ther. 2016, 23, 125–132. [Google Scholar] [CrossRef]
- Nakatsu, M.; Sainson, R.C.A.; Pérez-Del-Pulgar, S.; Aoto, J.N.; Aitkenhead, M.; Taylor, K.L.; Carpenter, P.M.; Hughes, C.C.W. VEGF121 and VEGF165 Regulate Blood Vessel Diameter through Vascular Endothelial Growth Factor Receptor 2 in an in vitro Angiogenesis Model. Lab. Investig. 2003, 83, 1873–1885. [Google Scholar] [CrossRef]
- Sörenby, A.K.; Kumagai-Braesch, M.; Sharma, A.; Hultenby, K.R.; Wernerson, A.M.; Tibell, A.B. Preimplantation of an Immunoprotective Device Can Lower the Curative Dose of Islets to That of Free Islet Transplantation—Studies in a Rodent Model. Transplantation 2008, 86, 364–366. [Google Scholar] [CrossRef]
- Song, S.; Kim, E.J.; Bahney, C.S.; Miclau, T.; Marcucio, R.; Roy, S. The synergistic effect of micro-topography and biochemical culture environment to promote angiogenesis and osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2015, 18, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, L.; Chang, C.C.; Nunes, S.S.; Williams, S.K.; Weiss, J.A.; Hoying, J.B. Manipulating the Microvasculature and Its Microenvironment. Crit. Rev. Biomed. Eng. 2013, 41, 91–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzypek, K.; Nibbelink, M.G.; Karbaat, L.P.; Karperien, M.; van Apeldoorn, A.; Stamatialis, D. An important step towards a prevascularized islet macroencapsulation device—Effect of micropatterned membranes on development of endothelial cell network. J. Mater. Sci. Mater. Med. 2018, 29, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lathuilière, A.; Cosson, S.; Lutolf, M.P.; Schneider, B.L.; Aebischer, P. A high-capacity cell macroencapsulation system supporting the long-term survival of genetically engineered allogeneic cells. Biomaterials 2014, 35, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Danielsen, N.; Bjursten, L.M. Reactive capsule formation around soft-tissue implants is related to cell necrosis. J. Biomed. Mater. Res. 1999, 46, 458–464. [Google Scholar] [CrossRef]
- Khosravi, N.; Maeda, A.; Dacosta, R.S.; Davies, J.E. Nanosurfaces modulate the mechanism of peri-implant endosseous healing by regulating neovascular morphogenesis. Commun. Biol. 2018, 1, 72. [Google Scholar] [CrossRef] [Green Version]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Valentin, J.E.; Ravindra, A.K.; McCabe, G.P.; Stewart-Akers, A.M. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng. Part A 2008, 14, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Sicari, B.M.; Badylak, S.F. Rethinking Regenerative Medicine: A Macrophage-Centered Approach. Front. Immunol. 2014, 5, 510. [Google Scholar] [CrossRef] [Green Version]
- Duffy, G.P.; Robinson, S.T.; O’Connor, R.; Wylie, R.; Mauerhofer, C.; Bellavia, G.; Straino, S.; Cianfarani, F.; Mendez, K.; Beatty, R.; et al. Implantable Therapeutic Reservoir Systems for Diverse Clinical Applications in Large Animal Models. Adv. Healthc. Mater. 2020, 9, 2000305. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levey, R.E.; Coulter, F.B.; Scheiner, K.C.; Deotti, S.; Robinson, S.T.; McDonough, L.; Nguyen, T.T.; Steendam, R.; Canney, M.; Wylie, R.; et al. Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device. Pharmaceutics 2021, 13, 2077. https://doi.org/10.3390/pharmaceutics13122077
Levey RE, Coulter FB, Scheiner KC, Deotti S, Robinson ST, McDonough L, Nguyen TT, Steendam R, Canney M, Wylie R, et al. Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device. Pharmaceutics. 2021; 13(12):2077. https://doi.org/10.3390/pharmaceutics13122077
Chicago/Turabian StyleLevey, Ruth E., Fergal B. Coulter, Karina C. Scheiner, Stefano Deotti, Scott T. Robinson, Liam McDonough, Thanh T. Nguyen, Rob Steendam, Mark Canney, Robert Wylie, and et al. 2021. "Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device" Pharmaceutics 13, no. 12: 2077. https://doi.org/10.3390/pharmaceutics13122077
APA StyleLevey, R. E., Coulter, F. B., Scheiner, K. C., Deotti, S., Robinson, S. T., McDonough, L., Nguyen, T. T., Steendam, R., Canney, M., Wylie, R., Burke, L. P., Dolan, E. B., Dockery, P., Kelly, H. M., Ghersi, G., Hennink, W. E., Kok, R. J., O’Cearbhaill, E., & Duffy, G. P. (2021). Assessing the Effects of VEGF Releasing Microspheres on the Angiogenic and Foreign Body Response to a 3D Printed Silicone-Based Macroencapsulation Device. Pharmaceutics, 13(12), 2077. https://doi.org/10.3390/pharmaceutics13122077