Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
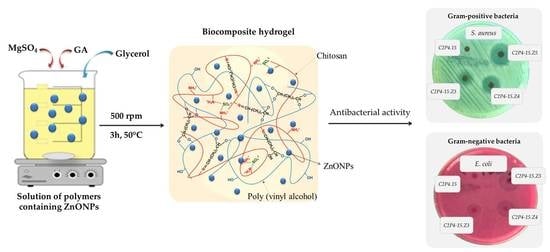
2.2. The Preparation of Biocomposite Hydrogels
2.3. Characterization of Biocomposite Hydrogels
2.3.1. The Structural Characteristics
2.3.2. The Morphological Characteristics
2.3.3. The Swelling Behavior
2.3.4. Tensile Mechanical Measurements
2.3.5. The In Vitro Cytotoxic Effects
2.3.6. The Antimicrobial Activities
3. Results and Discussions
3.1. Preparation of Biocomposite Hydrogels
3.2. Characterization of Biocomposite Hydrogels
3.2.1. The Structural Characteristics
3.2.2. Morphological Characteristics
ZnO Nanoparticles
Biocomposite Hydrogels
EDAX Analysis for Biocomposite Hydrogels
3.2.3. Swelling Behavior
3.2.4. Tensile Mechanical Measurements
3.2.5. The In Vitro Cytotoxic Effects
3.2.6. The Antimicrobial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Z.; Yue, L.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y.; et al. Antibacterial Hybrid Hydrogels. Macromol. Biosci. 2021, 21, 2000252. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Patil, J.S. Hydrogel System: An Approach for Drug Delivery Modulation. Adv. Pharmacoepidemiol. Drug. Saf. 2015, 4, e135. [Google Scholar]
- Peng, K.T.; Chen, C.F.; Chu, I.M.; Li, Y.M.; Hsu, W.H.; Hsu, R.W.W.; Chang, P.J. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 2010, 31, 5227–5236. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.L.; Hong, D.W.; Ku, K.L.; Lai, Z.T.; Chu, I.M. Novel thermosensitive hydrogels based on methoxy polyethylene glycol-co-poly(lactic acidco-aromatic anhydride) for cefazolin delivery. Nanomedicine 2014, 10, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J. 2014, 51, 182–190. [Google Scholar] [CrossRef]
- Glisoni, R.J.; Garcia-Fernandez, M.J.; Pino, M. Beta-Cyclodextrin hydrogels for the ocular release of antibacterial thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Oktar, F.N.; Mahirogullari, M.; Gunduz, O. Bone structure and formation: A new perspective. In Bioceramics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–193. [Google Scholar]
- Raţă, D.M.; Chailan, J.-F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—a promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316. [Google Scholar] [CrossRef]
- Baghaie, S.; Khorasani, M.T.; Zarrabi, A.; Moshtaghian, J. Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J. Biomater. Sci. Polym. Ed. 2017, 28, 2220–2241. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; Mansoori Moghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Ahmad Yusof, N.A.; Mat Zain, N. The Effect of ZnOP nanoparticles on the physical, mechanical, and antibacterial properties of Chitosan/Gelatin hydrogel films. J. Teknol. 2019, 81, 21–26. [Google Scholar]
- Raţă, D.M.; Cadinoiu, A.N.; Daraba, O.M.; Mihalache, C.; Mihalache, G.; Burlui, V. Metronidazole-loaded chitosan/ poly(maleic anhydridealt- vinyl acetate) hydrogels for dental treatments. Int. J. Med. Dent. 2016, 20, 92–97. [Google Scholar]
- Ahmadian, Y.; Bakravi, A.; Hashemi, H.; Namazi, H. Synthesis of polyvinyl alcohol/CuO nanocomposite hydrogel and its application as drug delivery agent. Polym. Bull. 2019, 76, 1967–1983. [Google Scholar] [CrossRef]
- Muppalaneni, S.; Omidian, H. Polyvinyl alcohol in medicine and pharmacy: A perspective. J. Dev. Drugs 2013, 2, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrieres, J.; Peptu, C.A. Chitosan/Poly (Vinyl Alcohol) Hydrogels for Entrapment of Drug Loaded Liposomes. Cellul. Chem. Technol. 2014, 48, 485–494. [Google Scholar]
- Jătariu, A.N.; Danu, M.; Peptu, C.A.; Ioanid, G.; Ibanescu, C.; Popa, M. Ionically and Covalently Cross-Linked Hydrogels Based on Gelatin and Chitosan. Soft. Mater. 2013, 11, 45–54. [Google Scholar] [CrossRef]
- Jătariu, A.N.; Holban, M.N.; Peptu, C.A.; Sava, A.; Costuleanu, M.; Popa, M. Double crosslinked interpenetrated network in nanoparticle form for drug targeting—Preparation, characterization and biodistribution studies. Int. J. Pharm. 2012, 436, 66–74. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Peptu, C.A.; Fache, B.; Chailan, J.F.; Popa, M. Microparticulated systems based on chitosan and poly (vinyl alcohol) with potential ophthalmic applications. J. Microencapsul. 2015, 32, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jatariu, A.N.; Popa, M.; Curteanu, S.; Peptu, C.A. Covalent and ionic co-cross-linking—An original way to prepare chitosan—Gelatin hydrogels for biomedical applications. J. Biomed. Mater. Res. Part A 2011, 98, 342–350. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [Green Version]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2018, 26, 154–174. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Dall′ Orto, V.C.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Daghdari, S.G.; Ahmadi, M.; Saei, H.D.; Tehrani, A.A. The effect of ZnO nanoparticles on infectious wounds. Nanomed. J. 2017, 4, 232–236. [Google Scholar]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. 2009. Available online: https://asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro (accessed on 25 September 2021).
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Dellali, K.Z.; Rata, D.M.; Popa, M.; Djennad, M.; Ouagued, A.; Gherghel, D. Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Eects in Breast Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 5659. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO nanoparticles: A study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Green. Process. Synth. 2019, 8, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 1, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozdoğan, A.; Aksakal, B.; Denktaş, C.; Salt, Y. Prestretching effect and recovery process of polyvinyl alcohol film crosslinked with tartaric acid. J. Appl. Polym. Sci. 2020, 137, e49421. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D.; Li, J.; Wang, K.; Wang, N. In-situ repair of graphene defects and enhancement of its reinforcement effect in polyvinyl alcohol hydrogels. RSC Adv. 2017, 7, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; Garcia, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2020, 82, 270–276. [Google Scholar]
- Abraham, A.; Soloman, P.A.; Rejini, V.O. Preparation of chitosan-polyvinyl alcohol blends and studies on thermal and mechanical properties. Proc. Techno. 2016, 24, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Chitosan/oxidized pectin/PVA blend film: Mechanical and biological properties. Polym. Bull. 2017, 74, 4297–4310. [Google Scholar] [CrossRef]
- Abdeen, Z.I.; El Faragy, A.F.; Negm, N.A. Nanocomposite framework of chitosan/polyvinyl alcohol/ZnO: Preparation, characterization, swelling and antimicrobial evaluation. J. Molec. Liq. 2018, 250, 335–343. [Google Scholar] [CrossRef]
- Pires, J.; de Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [Green Version]
- International Standard ISO 10993-5: 2009, Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; The International Organization for Standardization: Geneva, Switzerland, 2009.
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Pinho, A.R.; Martins, F.; Costa, M.E.V.; Senos, A.M.R.; da Cruz e Silva, O.A.B.; Pereira, M.d.L.; Rebelo, S. In Vitro Cytotoxicity Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells. Cells 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Kai, J.; Xuesong, Z. Preparation, Characterization, and Cytotoxicity Evaluation of Zinc Oxide–Bacterial Cellulose–Chitosan Hydrogels for Antibacterial Dressing. Macromol. Chem. Phys. 2020, 221, 2000257. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wang, R.; Yuan, P.; Fan, Z.; Yang, S. Preparation and property of ZnO/sodium alginate bi-layered hydrogel films as novel wound dressings. New J. Chem. 2019, 43, 8684–8693. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan. 2020, Mar 6, 457–489. [Google Scholar]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]











| Sample | CS/PVA Ratio (mg/mg) | GA/Free NH2 and OH Ratio (mol/mol) | MgSO4 / Free NH2 (mol/mol) | ZnO Nanoparticles in Relation to the Total Amount of Polymers (%) |
|---|---|---|---|---|
| C2P2.5 | 50/50 | 1/20 | 1/20 | 0 |
| C2P2.10 | 1/10 | |||
| C2P2.15 | 3/20 | |||
| C2P2.20 | 1/5 | |||
| C2P4.5 | 50/100 | 1/20 | ||
| C2P4.10 | 1/10 | 0 | ||
| C2P4.10.Z3 | 3 | |||
| C2P4.10.Z4 | 4 | |||
| C2P4.10.Z5 | 5 | |||
| C2P4.15 | 3/20 | 0 | ||
| C2P4.15.Z3 | 3 | |||
| C2P4.15.Z4 | 4 | |||
| C2P4.15.Z5 | 5 | |||
| C2P4.20 | 1/5 | 0 |
| Sample | C2P4.10 | C2P4.10.Z3 | C2P4.10.Z5 | C2P4.15 | C2P4.15.Z3 | C2P4.15.Z5 |
|---|---|---|---|---|---|---|
| Initial content of ZnONPs (w/w %) | 0 | 3 | 5 | 0 | 3 | 5 |
| Final content of ZnONPs (w/w %) | 0 | 2.09 | 4.63 | 0 | 2.14 | 4.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rata, D.M.; Cadinoiu, A.N.; Popa, M.; Atanase, L.I.; Daraba, O.M.; Popescu, I.; Romila, L.E.; Ichim, D.L. Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment. Pharmaceutics 2021, 13, 2079. https://doi.org/10.3390/pharmaceutics13122079
Rata DM, Cadinoiu AN, Popa M, Atanase LI, Daraba OM, Popescu I, Romila LE, Ichim DL. Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment. Pharmaceutics. 2021; 13(12):2079. https://doi.org/10.3390/pharmaceutics13122079
Chicago/Turabian StyleRata, Delia Mihaela, Anca Niculina Cadinoiu, Marcel Popa, Leonard Ionut Atanase, Oana Maria Daraba, Irina Popescu, Laura Ecaterina Romila, and Daniela Luminita Ichim. 2021. "Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment" Pharmaceutics 13, no. 12: 2079. https://doi.org/10.3390/pharmaceutics13122079
APA StyleRata, D. M., Cadinoiu, A. N., Popa, M., Atanase, L. I., Daraba, O. M., Popescu, I., Romila, L. E., & Ichim, D. L. (2021). Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment. Pharmaceutics, 13(12), 2079. https://doi.org/10.3390/pharmaceutics13122079